3 Laser-Assisted Nonsurgical Periodontal Therapy
Periodontal disease affects 80% of the adult population in the United States.1 Recent research suggests that bacteria associated with periodontal disease are associated with an increased risk of heart disease, diabetes, stroke, premature birth,2,3 and respiratory infection in susceptible individuals.4,5 Although periodontal disease thus should be one of the most frequently treated conditions in dentistry, traditional therapies have been poorly received, even feared, and viewed by many patients as “bad experiences.” Patients are reluctant to pursue initial periodontal treatment and are even more reluctant to have further treatment when disease progression occurs.
Periodontal Disease
Periodontal diseases are biofilm-initiated inflammatory conditions that impact susceptible individuals.6 Gingivitis, the first stage of periodontal disease, is defined as “gingival inflammation without loss of connective tissue attachment.”7 Periodontitis is defined as follows:
The presence of gingival inflammation at sites where there has been a pathological detachment of collagen fibers from cementum and the junctional epithelium has migrated apically.7 In addition, inflammatory events associated with connective tissue attachment loss also lead to the resorption of coronal portions of tooth-supporting alveolar bone.
Similar definitions are associated with periimplant mucositis and periimplantitis, respectively. The disease process is disrupted and controlled by host resistance through therapy or natural defenses.8
The organization and activity of biofilm are important because biofilm is the first component of periodontal disease targeted in therapy. Biofilm is a complex community of microorganisms protected by a secreted extracellular polymeric substance. As it becomes more mature, the microbes use a molecular communication, quorum sensing, to create a highly organized and adaptable infrastructure. The various microbes within the biofilm behave so as to preserve the entire community and become a living organism.9
As the biofilm responds to its environment, its adaptation provides resistance to such factors as ultraviolet (UV) light, bacteriophages, biocides, antibiotics, immune system responses, and environmental stresses.10 Manor et al.11 found that biofilm penetrates epithelium and underlying connective tissue, possibly to a depth of 500 microns (µ; or micrometers, µm). Biofilm has been observed penetrating tissues along the path of capillaries. Through various means, including stimulating the host’s inflammatory pathways, biofilm may control transudate production to supply its nourishment.12 This demonstrates the parasitic nature of biofilm in tissue.
As the body responds to biofilm invasion, proinflammatory cytokines, prostanoids, and proteolytic enzymes are synthesized and released. Fluids increase within the tissues, circulation becomes stagnant, swelling occurs, and metabolic products become backlogged. Enzymes such as collagenase, gellatinase, elastase, and fibrinolysin,3 so instrumental in the initial healing stage, remain at the site, destroying the developing strands of healing matrix needed to form connective tissue. The inflamed tissue is unable to progress from the granulation phase of healing into the remodeling phase because of the continued insult with pathogenic activities and further biofilm proliferation.13 The site is now a biofilm-infested chronic wound14 and, without treatment, will most likely progress, creating localized destruction and impacting systemic health.
Benefits of Laser Therapy
The advantages of lasers affect the bacteria directly and support the body’s healing response. Incorporating lasers into conventional therapies helps accomplish treatment objectives. Conventional nonsurgical periodontal therapy addresses debriding the area of bacteria, endotoxins, and hard deposits from the tooth structure to restore gingival health.8 Instrumentation is focused on the tooth structure and most often accomplished through manual and power scaling. In the future, lasers will also be used for root debridement.
As of this writing, the U.S. Food and Drug Administration (FDA) has not yet cleared laser-assisted removal of deposits and biofilm from tooth structure. However, Aoki et al.15 determined that deposits and biofilm are more thoroughly removed and that a more biocompatible surface is created for reattachment with an erbium (Er) laser versus conventional methods.16 The alexandrite laser also has been in development for selective removal of calculus from the root structure.17 The carbon dioxide (CO2) laser has been shown to increase adherence of fibroblasts to root surfaces, and the fibroblast adherence is superior to conventional techniques both in quantity of fibroblasts attached and in the quality of the attachment.18
Both in vitro and in vivo studies show that lasers are bactericidal.19–22 Although not specific to certain bacteria, the argon (Ar), neodymium-doped yttrium-aluminum-garnet (Nd:YAG), and diode lasers have strong absorption in darkly pigmented bacteria, causing a direct, increased effect on the red and orange–complex bacteria associated with periodontitis.23 The CO2 laser also has excellent bactericidal properties.24,25 Both CO2 and erbium lasers act on pathogens by heating intracellular fluids, causing the microbes to collapse.26,27 The absorption of laser energy by tissues produces a photothermal effect. With the appropriate parameters, most nonsporulating bacteria, including anaerobes, are readily deactivated at 50° C.28,29
In laser-assisted active phase I periodontal infection therapy, the diseased biofilm-infested tissues of periodontal pockets are debrided. Working within the recommended parameters of 60° C,30 the healthy tissue beneath the nonhealing granulation layer is not affected by the low energy. As noted earlier, biofilm can penetrate soft tissues. Removal of “bioburden” from the wound has a significant impact on wound bed preparation and wound healing.31 Steed et al.32 showed that more frequent debridement resulted in better healing than debriding less frequently. Moritz et al.19 reported that the bleeding index improved in 96.9% of the patients treated with laser-assisted periodontal therapy after conventional therapy, compared with 66.7% of patients treated conventionally without laser. The authors concluded that “the diode laser assisted periodontal therapy provided a bactericidal effect, reduced inflammation, and supported healing of periodontal pockets through elimination of bacteria.”19 Administering laser energy to the affected tissues at specific, repeated intervals is key in targeting biofilm during periodontal therapy.
Lasers also have the ability to seal capillaries and lymphatics, reducing swelling at the treated site and minimizing postoperative discomfort.33
Another benefit of laser-assisted procedures is the healing stimulated at the cellular level.34 Medrado et al.35 found that low-level laser treatment depresses the exudative phase while enhancing the proliferative processes during acute and chronic inflammation. Laser photobiomodulation can activate the local blood circulation and stimulate proliferation of endothelial cells.36,37 Wound healing is supported with reduced edema, polymorphonuclear (leukocyte) neutrophil (PMN) infiltrate, increased fibroblasts, more and better organized collagen bundles.38 Karu39 suggests these effects are caused by an increase in mitochondrial synthesis. The slight scattering that occurs with more deeply absorbed energy of certain lasers may have photobiomodulation effects beyond the direct application. The aiming beams of the lasers may also have a photobiomodulation effect (see Chapter 15). More research needs to be conducted in this area.
Laser Types
Laser wavelengths used to treat active phase I periodontal infection include the diode, Nd:YAG, CO2, and erbium lasers. Chapter 2 provides further information on each wavelength.
Argon Laser
The argon laser, emitting a 514-nm wavelength, was FDA cleared for sulcular debridement in 1991. The energy is delivered through a fiberoptic system in contact or noncontact mode, depending on the procedure. These wavelengths are highly absorbed in hemoglobin and melanin and have demonstrated bactericidal properties, particularly for Prevotella and Porphyromonas.23,40 However, argon lasers are no longer being marketed to dentists.
Diode Laser
The semiconductor diode lasers are available in four different wavelengths, as follows:
The 980-nm wavelength has more absorption in water than the other three diode wavelengths, which may be an added benefit to the laser interaction within the pocket. However, no definitive studies yet show that the superior absorption in water leads to superior clinical results compared with other diode wavelengths. Diode lasers are bactericidal19,41,42 and aid in coagulation.
Neodymium:YAG Laser
The Nd:YAG is a free-running pulsed laser. The laser energy is produced in bursts of photonic energy rather than a continuous beam. This laser also uses a fiberoptic delivery system for contact or noncontact procedures. The 1064-nm wavelength is most highly absorbed in melanin, less absorbed in hemoglobin, and slightly absorbed in water. The Nd:YAG is also bactericidal20 and provides excellent hemostasis. Because it is a free-running pulsed laser, the Nd:YAG emits high peak powers but allows for tissue cooling during the off time. When choosing settings for treatment, higher millijoules (mJ) with fewer repetitions per second (hertz, Hz) aids in coagulation, whereas lower mJ with higher Hz is typically used for decontamination.
Micropulsed CO2 Laser
The micropulsed 10,600-nm CO2 lasers incorporate the newest technology available in producing CO2 laser energy. Delivery by an articulated arm in noncontact mode facilitates treatment. A specific insert with a defocusing tip is used for administering laser energy within the periodontal pocket. This wavelength interacts with water and hydroxyapatite and has a depth of penetration of 0.5 mm. Inflamed tissue possesses increased water content and is therefore affected by the laser energy, as are crevicular fluids and intracellular fluids. Fluids are photothermally heated, then vaporized, with the cell membranes collapsing. The bacteria are inactivated,24,25 and dehydration occurs as energy is applied.
Erbium Family of Lasers
Erbium lasers are FDA cleared for both hard and soft tissue applications, and some Er lasers are FDA cleared for sulcular debridement. It is important to check with the manufacturer of the specific laser device to ensure that it is FDA cleared for its intended use. The Er:YAG 2940-nm and erbium, chromium–doped yttrium-scandium-gallium-garnet (Er,Cr:YSGG) 2780-nm instruments are free-running pulsed lasers that are most highly absorbed in water, followed by high absorption in hydroxyapatite and poor absorption in hemoglobin. The laser energy has a penetration depth of 5 µ. Thermal rise in the superficial tissue layers is minimal and even less with concurrent water spray during lasing. This limits hemostasis and coagulation within the pocket. Although some view the bleeding as a hindrance when working with these lasers, a benefit of this wavelength is almost no operative or postoperative discomfort. Quicker healing is also an advantage of these wavelengths.16,43 Studies have demonstrated significant population reduction of the periodontal pathogens Porphyromonas gingivalis and Actinobacillus (Aggregatibacter) actinomycetemcomitans, as well as positive long-term clinical results in attachment gain.16,21,26
Fundamentals of Laser Physics
Treatment Objectives with Soft Tissue Lasers
Sulcular Debridement with Fiberoptic Laser Delivery
Preprocedural Decontamination
Preprocedural decontamination is a laser application done before any instrumentation, even probing. The objectives are to affect the bacteria within the sulcus, reducing the risk of bacteremia caused from instrumentation, and to lower the microcount in aerosols created during ultrasonic instrumentation.44 The technique uses very low energy. The fiber is placed within the sulcus and is swept vertically and horizontally against the tissue wall, away from the tooth, with a smooth, flowing motion, for 7 to 8 seconds on the lingual aspect, then on the buccal of each tooth’s tissue wall. The benefits of preprocedural decontamination are seen in the reduced microbial translocation through the circulatory system.
Decontamination
Just as conventional root debridement removes biofilm and accretions from the hard tooth surface, laser decontamination removes biofilm within the necrotic tissue of the pocket wall. The laser energy interacts strongly with inflamed tissue components (from preferential absorption by chromophores, which are more abundant in diseased tissues) and less strongly with healthy tissue. This nonsurgical therapy uses very low settings and decontaminates rather than cuts the tissue.28
The administration of laser decontamination requires an understanding of current periodontal pocket topography, proficiency in technique, and recognition of laser-tissue interaction to determine the end point of therapy. An updated periodontal chart is needed for reference throughout therapy. With conventional root debridement provided just before laser decontamination, the pocket depths may need to be reprobed for accuracy of laser treatment. Laser therapy should address sites presenting with inflammation and/or pocketing of 4 mm or greater. It is helpful to visualize the surface area being treated. For example, the diseased pocket lining requiring treatment in a case with 50% generalized bone loss has an estimated tissue surface area of 40 cm2 (6.2 in2), and in a case with 4 to 5 mm of generalized pocketing, 20 cm2 (3.1 in2).45 Knowing the depth and shape of pockets and area to be addressed enables the clinician to be thorough with technique.
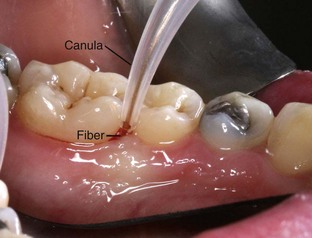
With fiberoptic technique, the fiber tip engages every millimeter of the diseased tissue wall that corresponds to the indicated sites marked on the periodontal chart. To begin, calibrate the fiber exposed to measure 1 mm less than the pocket depth (Figure 3-1). Begin lasing with the fiber placed just inside the gingival margin, progressing apically, or place the fiber 1 mm short of the depth of the pocket, working coronally (Figure 3-2). The edge of the canula indicates the treatment depth of the pocket being reached. Use a rapid, gliding, multidirectional motion with the tip of the fiber in constant contact with the pocket wall. The strokes should address small sections and should be systematically overlapping. It is helpful to divide the pocket into affected sections, such as interproximal to the line angle, the direct buccal or lingual surface, and the line angle to the interproximal area. Continually inspect the fiber tip and remove any accumulated debris (Figure 3-3) with water-moistened gauze.
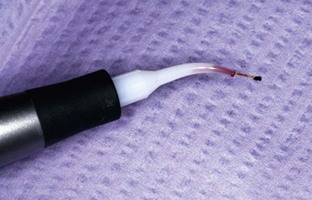
When exiting the pocket, take care to inactivate the laser, preventing overexposure of the gingival margin’s thin tissue. If the fiber tip becomes irreversibly coated (Figure 3-4), cleave the fiber, calibrate the length of the pocket, and continue the procedure.
FIGURE 3-4 • Excessive amount of debris on fiber. At this stage of treatment, the fiber should be cleaved and recalibrated.
Completion of laser decontamination is determined by laser parameters used, delivery time, and clinical signs. Decontamination is accomplished with less mJ and more Hz than for coagulation. A more inflamed pocket may require less average power because of increased concentration of the laser’s preferred chromophores. If working with an initiated fiber and lower settings in continuous wave, interaction is concentrated and will require less treatment time than a noninitiated fiber with higher settings in pulsed mode. A deeper pocket will require longer treatment time because of increased surface area. As lasing progresses in an area, less and less debris should collect on the fiber. Fresh bleeding will occur, however, when the pocket wall is fully decontaminated and debrided (Figure 3-5). Keep in mind the laser parameters and application time. Observing tissue interaction is essential in determining how long a diseased site should be treated.
FIGURE 3-5 • Gingiva immediately after fiber-based laser treatment, showing fresh bleeding from the pocket.
Sulcular Debridement with CO2 Laser
Begin by directing laser energy to the coronal edge of the marginal gingiva. Hold the tip perpendicular to the tissue crest at a distance of approximately 1 mm. Tissue interaction is observed with a slight “frosting” of the surface (Figure 3-6). Marginal dehydration will improve entry of the tip by drawing the tissue slightly away from the tooth structure. The epithelium will be inhibited by this application. This is the first step before pocket decontamination.
The technique for decontamination involves placement of the laser’s defocusing tip 1 to 2 mm into the pocket (only 1 mm for pockets up to 6 mm in depth; only 2 mm for pockets >6 mm). Treatment should include the complete circumference of each tooth presenting with disease. Activate the laser as the tip is drawn through the crevicular space in an even, slow motion, working from the distal aspect to the mesial aspect on the buccal and again on the lingual side of the tooth. The laser tip is kept parallel to the long axis of the tooth (Figure 3-7).
Treatment time is a maximum of 16 seconds per buccal or lingual surface. The length of application time depends on the extent of disease and the surface area; larger teeth such as molars are treated longer than smaller teeth such as lower anteriors.46 The tip must be kept open and free of coagulum for efficient energy flow. Keeping the tissue slightly moist and working in a single direction will enhance the laser’s efficiency. Vertical up-and-down movements or pushing the tip toward the tissue can cause the tip to clog. If the tip becomes occluded, the tip and detritus inside will continue absorbing the energy, heating the tip excessively. Coagulation occurs simultaneously with decontamination. No additional steps are necessary in lasing with the CO2 laser. Figure 3-8 shows the gingiva after-lasing.
Postoperative Care
Postlaser irrigation is a subject of debate. Although irrigation with chlorhexidine or other solutions is used in conventional treatment as a final step in disinfecting periodontal pockets, the author believes that postlaser irrigation is unnecessary. In fact, Mariotti and Rumpf47 found that solutions of chlorhexidine (≤0.12%) in contact with wound sites for even a short time could have serious toxic effects on gingival fibroblasts. Other studies report that subgingival irrigation has no significant additive effects on periodontal healing.48–50
The final step in postoperative care is advising the patient on what to expect, addressing further concerns, and discussing continued self-care. Counsel the patient that mild discomfort is possible the first 24 to 48 hours. With laser-assisted nonsurgical periodontal therapy, discomfort is often associated more with root debridement than lasing. Excessive pain may indicate another issue and should be evaluated. The patient should avoid spicy, sharp, and crunchy foods for 24 hours to avoid discomfort and trauma. Seeds and husks may become lodged between the gum and tooth and should be avoided. The risk of a foreign object impeding healing is highest in the first few postoperative days, but risk may persist if the case is more severe. Encourage the patient to be diligent in supporting the healing process by consistent and thorough daily cleaning. Patients appreciate instructions in written form for reference as well as in verbal form (Box 3-1).
Box 3-1 Patient Care Instructions after Laser-Assisted Periodontal Therapy
Healing and Tissue Rehabilitation
Healing typically occurs without complications as the body responds to therapy. Through the first 24 to 72 hours, the patient may feel tenderness and experience light bleeding when eating or cleaning; after tooth debridement and tissue decontamination, epithelium begins to regenerate after 24 hours, then progresses 1 mm per day, protecting the tissue wall. Within 1 week, the wound surface is covered. Connective tissue begins proliferation by day 5. Because laser decontamination procedures are repeated at 10-day intervals, the epithelium is impaired during laser biofilm reduction. This allows the fibroblasts to continue organizing into a connective tissue attachment apparatus. The attachment will continue to mature for 12 months.8 Because this attachment is easily disrupted, only light-pressure probing is recommended after several months and normal probing after 6 months of postoperative therapy.51
Classic signs of tissue rehabilitation include improved color, consistency, texture and stippling, and contour. These signs, as well as a decrease or elimination of hemorrhaging during gentle tissue manipulation and a reduction of pocket depth, are all desired indications of healing tissues. Figure 3-9 shows initial periodontal probing before treatment and periodontal probing 6 months after treatment. Figure 3-10 shows three sets of probings: initial probing, 8 weeks after treatment, and 5 months after treatment. Analysis of the probing results shows a resolution of 86% of the bleeding sites, a decrease in 86% of the pocketing sites, and a 58% decrease in the number of teeth exhibiting periodontal disease.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses