CHAPTER 3 Immediate Loading
Immediate loading of implants has been reported in the literature by several authors. In 1997, Schnitman1 and Balshi,2 in two separate studies of immediate-loading implants, reported surgical success rates of 85% and 80%, respectively. Following the development of the Brånemark Novum protocol, Brånemark and co-workers3 reported a 98% success rate in 1999; in 2004, van Steenberg and co-workers4 reported a 93% success rate. The changes in both the surgical and prosthetic protocols are impressive and are a significant departure from the original two-stage protocol. To better understand the significant changes in the immediate-load protocol, it is prudent to understand the biologic bases for this approach.
The two-stage protocol used a machined-surface implant made from commercially pure titanium. The texture and the condition of the implant surface5—specifically the outermost atomic layer—is a key factor in osseointegration. Implant surfaces vary based on the handling and preparation methods used.6 The composition of commercially pure titanium is 99.7% titanium, 0.05% iron, 0.10% oxygen, 0.03% nitrogen, 0.01% carbon, and 0.06% of other elements.7–9 It is milled in an argon-gas environment. After completion of the milling process, its exposure to air forms a 50- to 100-Å-thick oxide layer.7–10 The oxide layer of osseointegrated implants is surrounded by glycoprotein with an adjacent calcified layer approximately 100 Å in width. For the machined implant to establish an intimate contact between the titanium oxide, the glycoprotein, and the calcified matrix, the process of osseointegration or distant osteogenesis requires several weeks of undisturbed healing time. The concept of distant osteogenesis11 is discussed in more detail in the following section.
Immediate loading of implants has been facilitated in part by the modification of the implant surface, generally referred to as rough or microtextured surfaces. This modification has led to higher predictability when adopting the immediate-load concept. Reports of 98% surgical success have been published using the TiUnite surface.12–14 These findings are consistent with compositional changes of the implant surface influencing biocompatibility and healing.15 Rough surfaces influence osseointegration by (1) increasing the implant surface area within the bony osteotomy,16 (2) enhancing initial stability by fibrin matrix retention,17 and (3) “accelerating” osseointegration by their osseoconductive properties.18,19
The methods for enhancing machined implant surfaces are the application of titanium plasma spray or performing anodic oxidation.19 Recent literature indicates that the anodic oxidation process, such as is used with the TiUnite implant surface, seems to have higher success rates.20,21 Such success is in part due to the osseoconductive properties of the roughened surface, which results in better fibrin clot retention on the implant surface and initiates contact osteogenesis over the implant surface.11 Better primary stability of roughened surfaces versus machined surfaces has been demonstrated in dog models.22 In 2001, Glauser23 also demonstrated the superior stability of textured implant surfaces over machined implant surfaces using resonance frequency analysis. The stability of implants was measured during bone healing as well as at the end of the osseointegration period. Throughout the study, the textured implant had higher implant stability quotient (ISQ) values compared with the machined implants.23
It is apparent that the biologic bases of immediate loading are correlated with the topography of the implant as well as with its surface morphology. The composition of the implant, limiting micromotion as described by professors Brunski and Skalak,60 and using a rigid framework during the healing period are all compatible with the biologic healing cascade of bone.
Contact Osteogenesis versus Distant Osteogenesis
This section discusses bone biomechanics and biology as described by Davis.11 Upon initiation of an osteotomy (trauma) onto the surface of the alveolar bone, damage to the intraosseous blood vessels occurs with resultant bleeding into the osteotomy site of approximately of 1 ml (Figure 3-1).11 Extravasations of blood during trauma to long bones and resultant loss of approximately 1 L of blood has been reported by others.21 This vascular disruption on the surface of the osteotomy site during preparation of the implant site leads to death of the peri-implant cortical bone.24
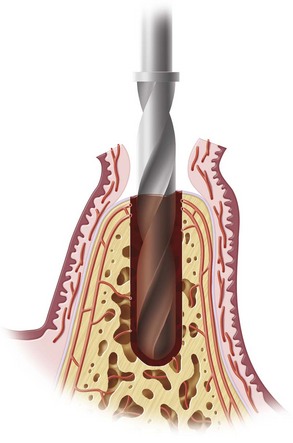
FIGURE 3-1 Approximately 1 ml of blood fills the osteotomy cavity during preparation for implant placement.
The healing at this site is initiated by osteoclastic activity from the medullary compartments underlying the affected cortical bone.25 This phenomenon has been called osseointegration, as described by previous reports.26,27
The macrostructure of bone is composed of trabecular (cancellous) and cortical (compact) portions. The function of cortical bone is to withstand torsional loading, whereas cancellous bone has evolved to predominantly withstand compressive loading. These differences in the macrostructure of bone are clearly seen when evaluating the macrostructure of long bones. The diaphysis is made of primarily cortical bone and the articular extremities are made of primarily cancellous bone.11 These two differences in the macroarchitecture of bone are also present in the maxillofacial region and have been shown to formulate a clinical classification of bone type in the edentulous maxilla and mandible.28
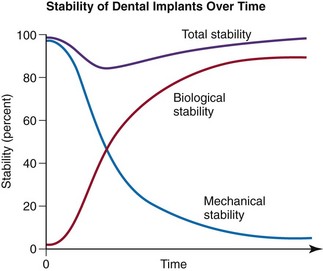
The microarchitecture of cortical and cancellous bone includes 3% to 5% of both bone types that are continuously being resorbed and replaced by osteoclasts and osteoblasts, respectively.29,30 Both types of bone are needed to allow the initial mechanical stability of the cortical bone to stabilize the implant while the biologic stability of the cancellous bone matures (Figure 3-2). This phenomenon of mechanical stability giving way to biologic stability has been studied by Glauser and others31–35 using resonance frequency analysis.
Laying down new bone on the walls of the osteotomy site and the implant surface itself are termed distant and contact osteogenesis, respectively.11,36 Distant osteogenesis occurs on cortical bone surfaces when new bone forms on the surface of old bone lining the walls of the implant osteotomy site. In contact osteogenesis, new bone is formed for the first time directly on the implant surface.
Contact osteogenesis—new bone formation on the surface of the newly placed implant within the osteotomy by differentiating osteogenic cells—is referred to as de novo bone formation.37,38 The presence of a cement line matrix, as described by von Ebner39 and Davis,11 is the hallmark for the histologic appreciation of this concept.
Both distant and contact osteogenesis occurs at every endosseous implant osteotomy healing site. The initial stability31,40 attained by the cortical aspect of the bone is crucial to allow recruitment of osteogenic cells onto the implant surface, initiating contact osteogenesis. Recruitment of the osteogenic cells to the implant surface is related to implant surface design and surface texture.
Contact Osteogenesis
Extravasated blood within the osteotomy site has a composition as outlined in Figure 3-3. Red blood cells are obviously important in oxygen transportation, but do not have a major influence in early bone healing. Platelets, however, play a major role in the mechanism of early bone healing through their degranulation and release of platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β), as well as through vasoactive factors such as serotonin and histamine, which play a major role in regulation of wound healing cascade.41,42 Stimulation and proliferation of human osteoblasts by PDGF is extensively reported in the literature.43,44 PDGF and TGF-β are mitogenic and chemotactic factors for fibroblasts, neutrophil, and osteogenic cells.45–47 Platelet degranulation also results in the release of platelet releasate, which also plays a role in the stimulation, recruitment, migration, and proliferation of bone marrow–derived cells.48
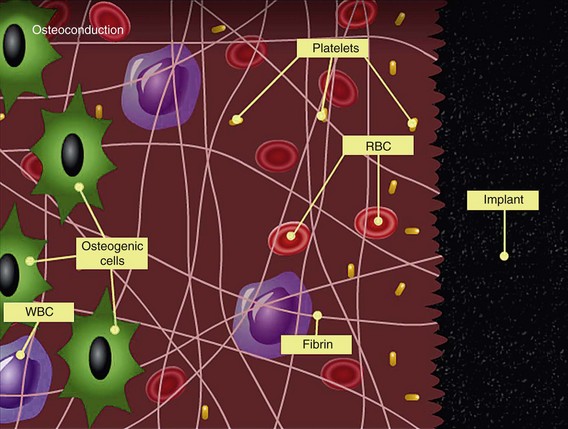
FIGURE 3-3 Composition of various cells found in extravasated blood within the osteotomy site.
(Courtesy Dr. John E. Davies, University of Toronto.)
Contact osteogenesis begins with activation of platelets and their contact with the implant surface. Glycoprotein (GP) IIb/III integrin binds to the implant surface fibrinogen21 and mediates the adhesion of platelets. Greater platelet adhesion is seen on implants that have surfaces of greater porosity.49 Platelets activated by contacting the implant surface release cytokines, growth factors, and microparticles, which, in conjunction with the three-dimensional matrix formed by the fibrin in the coagulation cascade, results in the migration of osteogenic cells to the implant surface.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses