3 Endodontic Microbiology
Since the pioneering studies of Miller (1894), there has been continuous interest in the microbiologic aspects of endodontic diseases. Almost all clinical situations requiring endodontic treatment have a microbiologic etiology or may later develop a microbiologic component. Subsequently, to ensure the best possible chance of success in treatment, the dentist must be familiar with the key aspects of endodontic microbiology:
• the role of microbes in the etiology and pathogenesis of the various diseases involving the tooth root canal system;
• the effect of the treatment procedures on the microbial infection;
• methods of preventing reinfection of the root canal.
3.1 Detection of bacteria
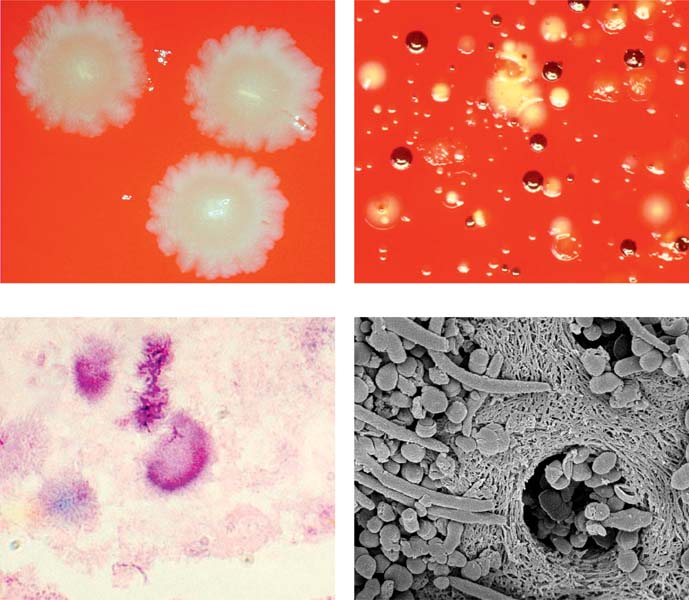
Primary apical periodontitis: infection by anaerobic bacteria. Anaerobic and microaerophilic bacteria are the key microbial components of primary apical periodontitis. These images show some of the most important microorganisms causing endodontic disease.
Above left: Fusobacterium nucleatum colonies.
Above right: Prevotella intermedia (dark brown) and Porphyromonas gingivalis (light brown) colonies together with colonies of other bacteria from an infected root canal.
Below left: Histologic preparation showing bacterial colonies in periapical actinomycosis.
Below right: Scanning electron microscopic image of bacteria penetrating into dentinal tubules.
Etiology of Apical Periodontitis
The connection between microbes and apical periodontitis (AP) is well established. Using gnotobiotic rats (rats raised in a sterile environment), Kakehashi et al. (1965) were the first to demonstrate the indisputable causal relationship between periapical inflammation (apical periodontitis = lesion of endodontic origin [LED]) and the presence of microbes in the root canal. Compared with normal rats, gnotobiotic animals with pulps exposed to the oral cavity and saliva with no bacteria developed no periapical disease.
Kakehashi and coworkers’ results were verified and refined further by Bergenholtz (1974) and Sundqvist (1976), who showed that intact necrotic human teeth exhibited apical periodontitis only if bacteria were present in the root canal, whereas necrotic teeth with a sterile root canal exhibited no periapical pathoses.
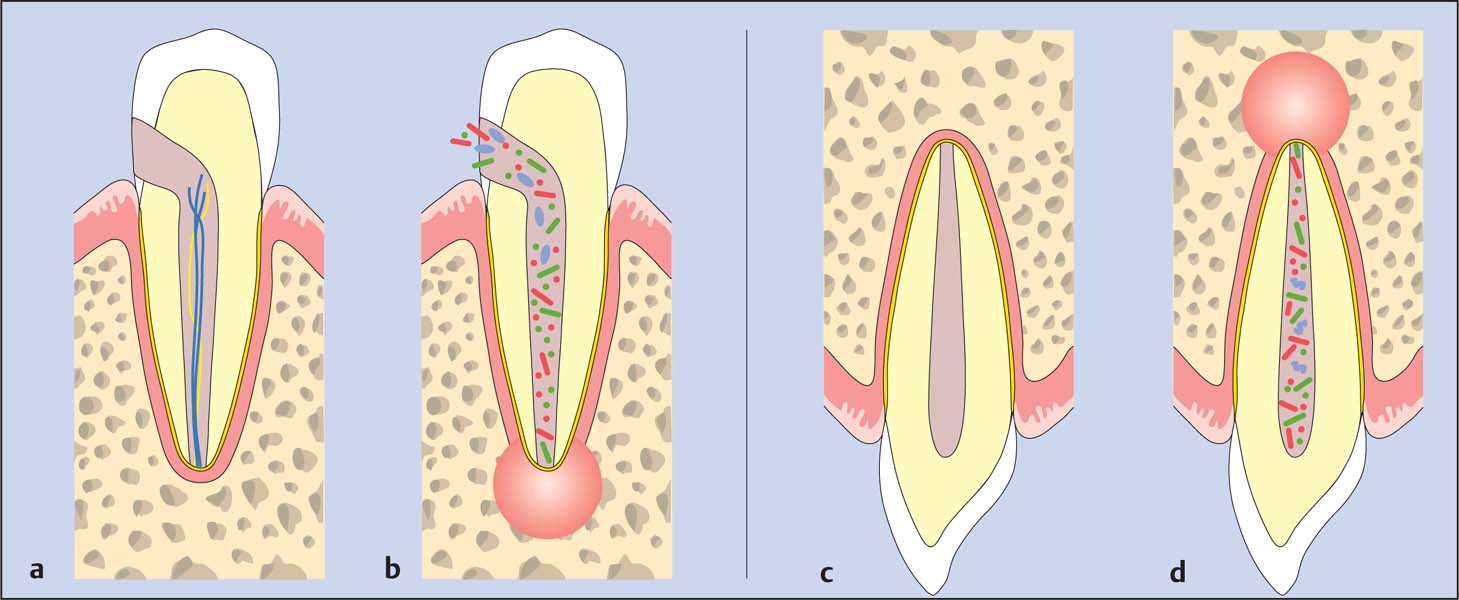
3.2 Classic studies of periapical periodontitis
Kakehashi et al. (1965) demonstrated that pulpal exposure without bacteria did not lead to pulpal necrosis in gnotobiotic rats (a). However, as soon as experimental animals came into contact with rats with normal oral bacterial flora, radiographs revealed pulpal necrosis and periapical lesions shortly after (b). Sundqvist (1976) studied intact but necrotic teeth with and without periapical lesions. Periapical lesions could only be detected in teeth in which bacteria could be identified in the root canals and subsequently cultured (c, d).
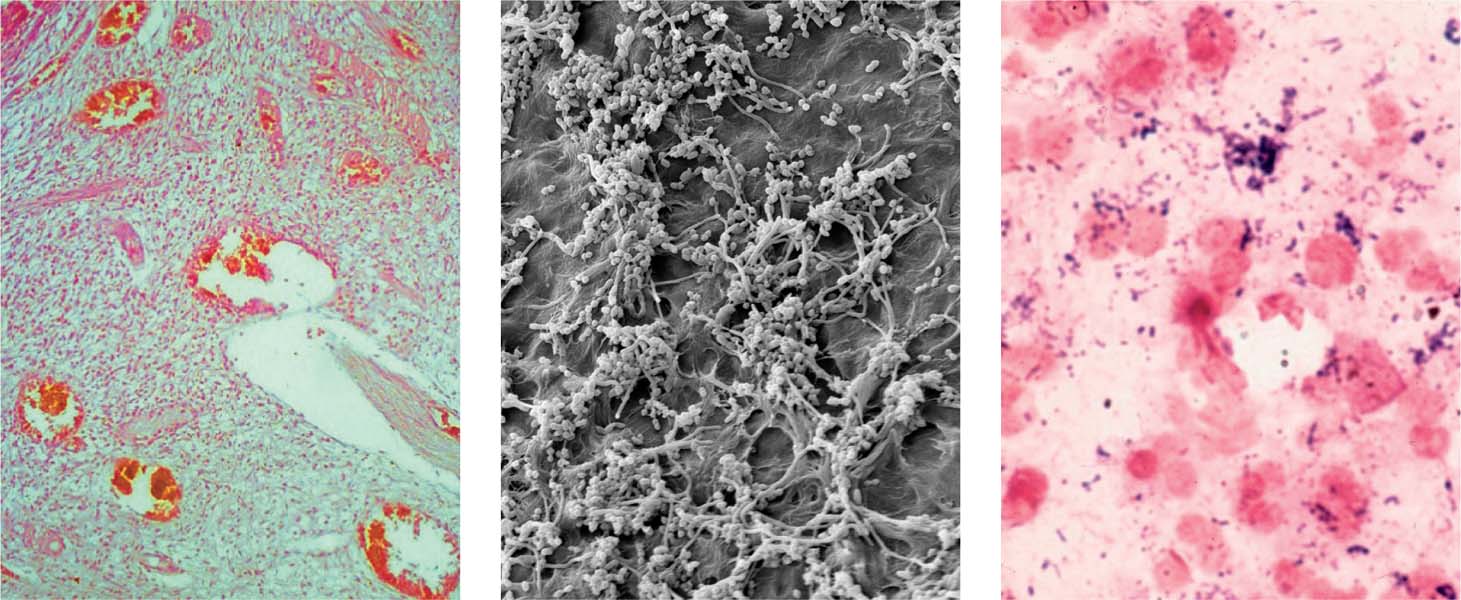
3.3 Bacterial infection is the cause of apical periodontitis
Left: Inflamed area within the coronal pulp. Although bacteria have not yet progressed into the pulp, an initial inflammatory reaction appears.
Middle: The biofilm within the root canal contains bacteria (cocci and rods) in dense aggregates showing various morphotypes.
Right: Gram-stained biopsy from the necrotic root canal of a tooth with associated AP; note the relatively even distribution of numerous leukocytes (red) Gram-positive cocci, and rod-like bacteria (blue).
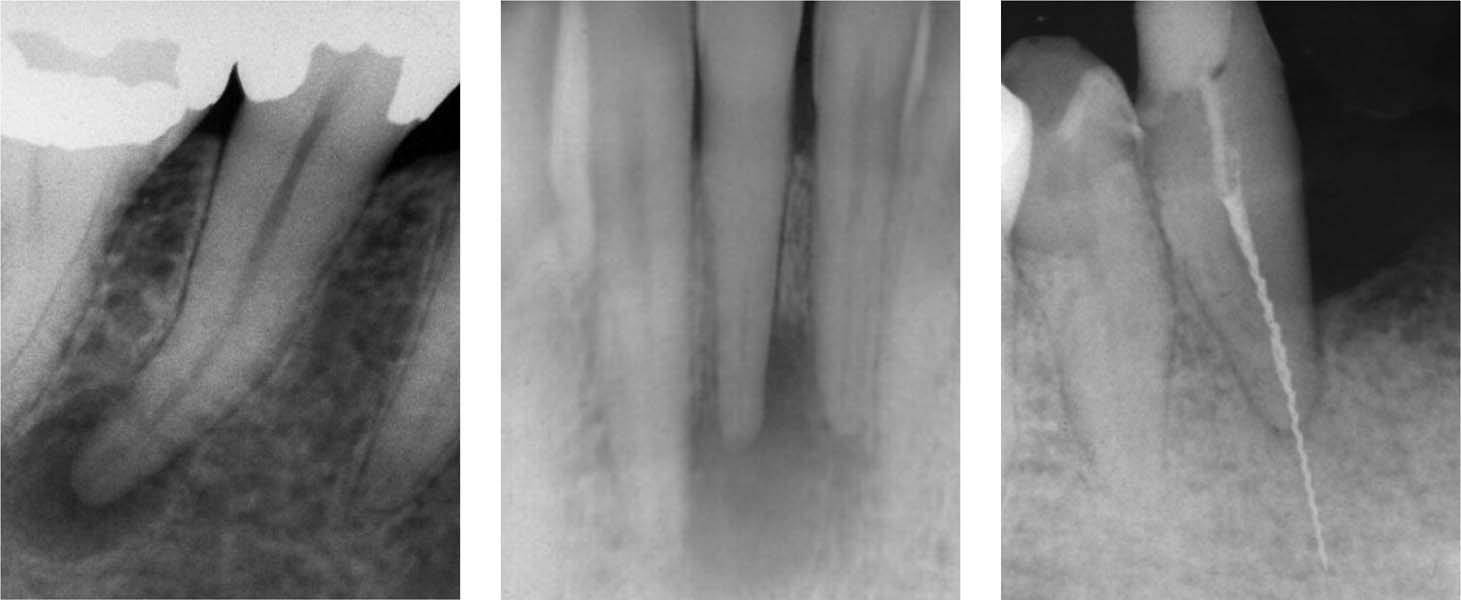
3.4 Radiographic appearance
Left: The radiolucent periapical lesion in this necrotic tooth indicates the presence of an infection in the necrotic root canal.
Middle: AP in an apparently intact tooth. The etiology in such a case is usually trauma, which leads to necrosis. Bacteria penetrate through microscopic canals and lateral canals, or as bacteremia into the root canal.
Right: A fractured H file in a tooth with a partially filled root canal; the file extends far beyond the apex. The radiograph shows no evidence of an apical lesion.
Pathogenesis of Pulpitis and Apical Periodontitis
The initial immunologic reactions in the development of pulpitis occur in response to bacterial antigens released from the advancing bacterial front within the carious lesion. These antigens are first “examined” by the antigen-detecting/antigen-presenting cells (APCs) of the pulp.
Dendritic cells are the main APCs in the pulp and macrophages (except histiocytes) which have major histocompatibility complex (MHC) class II molecules on their cell surface (Jontell et al., 1998). The class II molecule is a gene product of MHC and present foreign material (e.g., antigens) to T cells in the regional lymph nodes for recognition.
Following this, memory T cells are released into the circulation in the tissues, including the pulp, where they contribute to the secondary immune response. When the microbial cells in the advancing front of the carious lesion enter the pulp, infiltration of polymorphonuclear leukocytes and other inflammatory cells such as T memory cells and plasma cells can be observed.
Immunologic defense reactions anywhere in the body cause local tissue destruction, and the pulp is no exception. Micro-abscesses and focal necrosis can sometimes be seen in the pulp even before the carious lesion (and the microorganisms) invades the pulp.
With progression of caries and increasing antigen challenge, the area of necrosis increases in size and eventually the entire coronal pulp becomes necrotic. It is only at this stage that the root canal(s) of the affected tooth will have become infected.
Acute inflammatory responses to advancing infection in the periapical area involve numerous cells of the host inflammatory response system, and are induced by endogenous mediators such as prostanoids, kinins, and neuropeptides. Interleukin-1 and prostaglandins are key mediators of periapical bone resorption by osteoclasts (Stashenko et al., 1998).
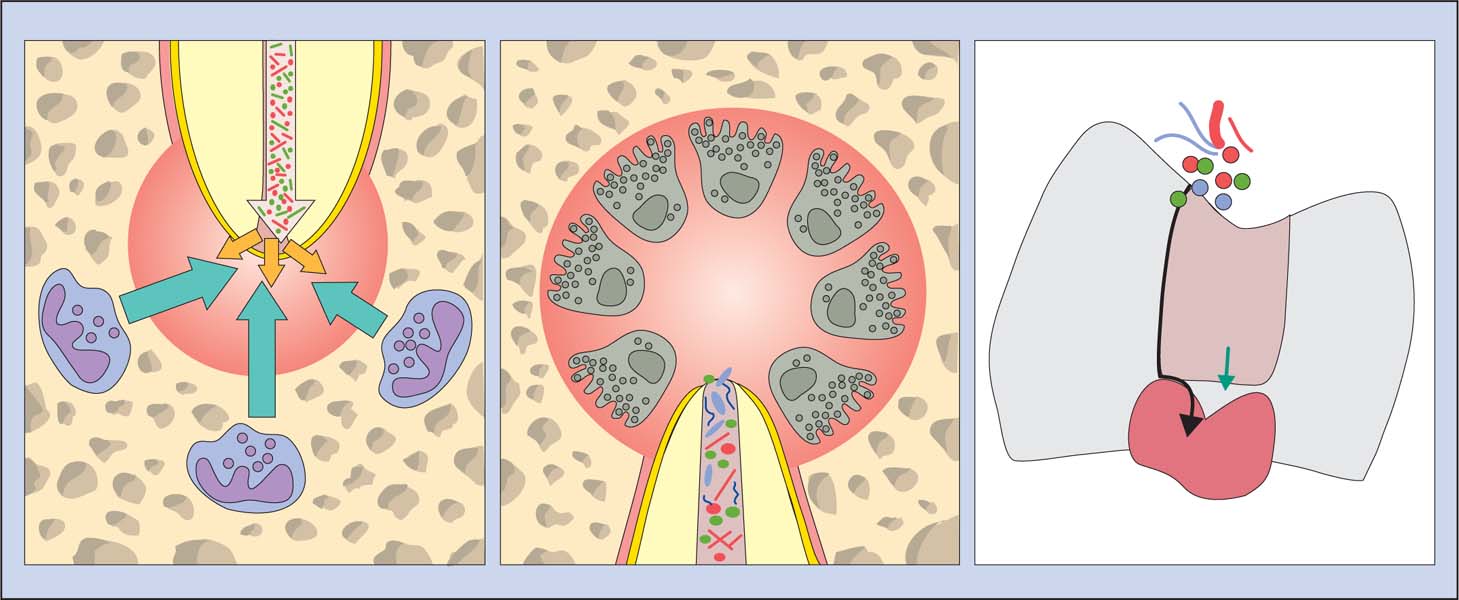
3.5 Immune reaction
Left: Stability in the periapical region: The immune defense cells inhibit an invasion of microorganisms from the necrotic root canal.
Middle: In a case of chronic AP, the osseous tissue is resorbed by multinucleated osteoclasts that are activated by an immunologic cascade.
Right: The pulp tolerates the chemical assault of numerous filling materials (green arrow). Microbial leakage through the space between tooth and root canal filling material (black arrow) often lead to pulpal irritation.
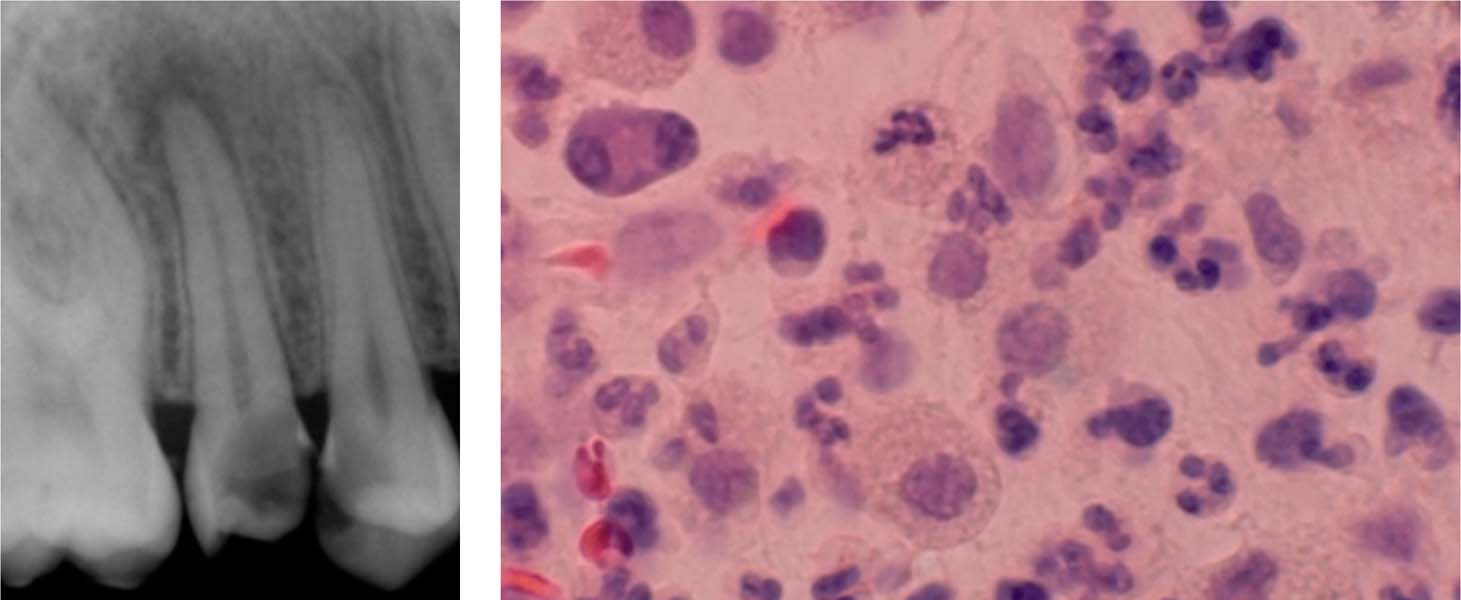
3.6 Apical periodontitis
Left: A radiograph showing a deep carious lesion in a maxillary premolar. The maxillary second premolar also has a periapical lesion representing AP.
Right: Granulocytes, plasma cells, and lymphocytes are apparent in this histologic section of this periapical region of a tooth with chronic AP.
Microbiota in Primary Apical Periodontitis
Chronic Apical Periodontitis
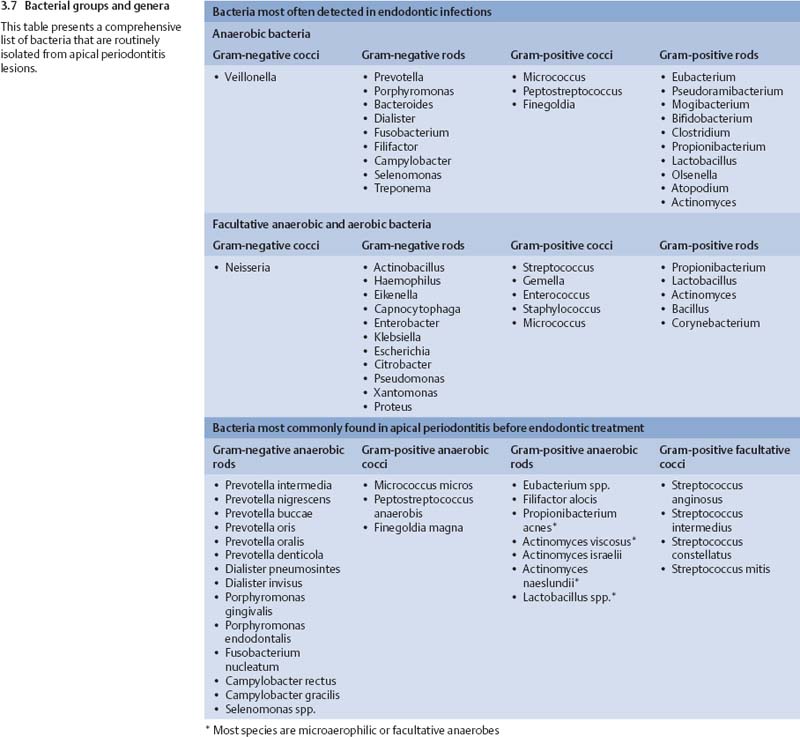
Chronic apical periodontitis is a symptom-free apical inflammation caused by microbes within the necrotic root canal. There is usually polymicrobial infection, dominated by obligate anaerobic bacteria (Moller et al., 1966; Bergenholtz 1974; Kantz and Henry, 1974; Sundqvist, 1976, 1994; Heimdahl et al., 1985; van Winkel-hoff et al., 1985, 1988; Haapasalo et al., 1986a, b; Sund qvist et al., 1989; Haapasalo 1993; Siqueira et al., 2001; Fouad et al., 2002; Khemaleelakul et al., 2002; Munson et al., 2002; Siqueira and Rocas, 2003, 2004a).
The number of different species per case is relatively small, typically two to eight species, with rarely more than 15 species in one tooth. The most frequent isolates are Dialister pneumosintes (Bacteroides pneumosintes), Tannerella forsythensis (B. forsythus), Prevotella spp., Porphyromonas spp., Fusobacterium spp., Treponema spp., Campylobacter rectus (Wolinella recta), Micromonas micros (P. micros), Eubacterium spp., Bifidobacterium spp., Actinomyces spp., Propionibacterium spp., Lactobacillus spp., and Streptococcus spp. Most of the above genera are strictly anaerobic, some include both anaerobic and facultative anaerobic species and strains, while streptococci are facultative bacteria. E. faecalis is less often observed in primary apical periodontitis than in posttreatment endodontic disease (Peciuliene et al., 2001; Rocas et al., 2004). Bacteria most frequently isolated from primary apical periodontitis lesions are shown in Figs. 3.7 and 3.8.
Acute Apical Periodontitis
Clinical signs and symptoms of acute apical infection include pain, swelling/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses