Luting agents
Introduction
For the greater part of the 20th century, the only materials available for the retention and marginal seal of fixed prostheses such as veneers, inlays, crowns and bridges, were zinc oxide–eugenol and zinc–phosphate cements. Hence, the term cementation represented an appropriate description of the process of fixing a metallic or ceramic restoration to the teeth. However, in the last quarter of the 20th century, things began to change with the introduction of many more adhesive materials and procedures. A wide variety of new cements have become available, such as zinc–polycarboxylate cements, glass–ionomer cements (GICs) and resin-modified glass–ionomer cements (RMGICs). There is now also a growing market for resin adhesive technologies.
In this context, the term ‘cementation’ hardly does justice to the range of materials now in use. Another term for the process of fixing a restoration in place is luting. The word ‘lute’ means a cement or other material used as a protective covering or an airtight stopping. As this term is not specific to a cement, the term luting agent perhaps provides a more appropriate description of some of the materials that are used today, such as the resins.
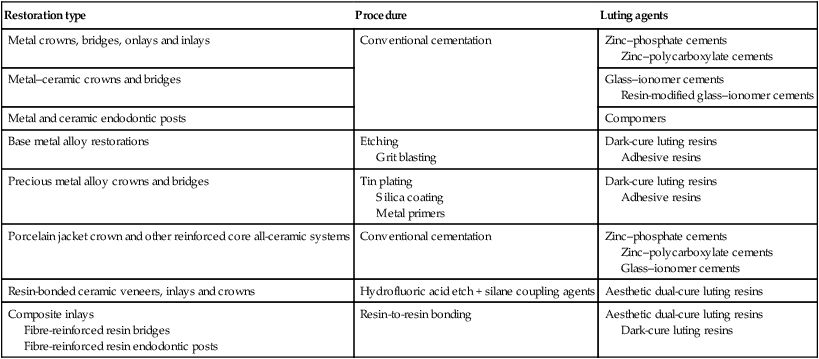
Whatever the relative merits of the terminology used, this chapter is concerned with the materials used for the permanent retention of posts and indirect restorations, as outlined in Table 3.8.1.
Table 3.8.1
Range of procedures and luting agents provided for the cementation of indirect restorations
| Restoration type | Procedure | Luting agents |
| Metal crowns, bridges, onlays and inlays | Conventional cementation | Zinc–phosphate cements Zinc–polycarboxylate cements |
| Metal–ceramic crowns and bridges | Glass–ionomer cements Resin-modified glass–ionomer cements |
|
| Metal and ceramic endodontic posts | Compomers | |
| Base metal alloy restorations | Etching Grit blasting |
Dark-cure luting resins Adhesive resins |
| Precious metal alloy crowns and bridges | Tin plating Silica coating Metal primers |
Dark-cure luting resins Adhesive resins |
| Porcelain jacket crown and other reinforced core all-ceramic systems | Conventional cementation | Zinc–phosphate cements Zinc–polycarboxylate cements Glass–ionomer cements |
| Resin-bonded ceramic veneers, inlays and crowns | Hydrofluoric acid etch + silane coupling agents | Aesthetic dual-cure luting resins |
| Composite inlays Fibre-reinforced resin bridges Fibre-reinforced resin endodontic posts |
Resin-to-resin bonding | Aesthetic dual-cure luting resins Dark-cure luting resins |
General requirements for luting agents
Biocompatibility
When luting agents are used in such situations as crowns and inlays, the material will inevitably be in contact with a relatively vast surface area of dentine. Hence, their susceptibility to producing postoperative sensitivity or pulpal inflammation is a very important consideration. Luting agents will also provide the main barrier to the ingress of bacteria, such that, besides a good marginal seal, possible antibacterial properties may prove to be highly beneficial.
Retention
The primary role of the luting agent is to provide retention of the restoration. With the water-based cements, such as zinc–phosphate cement, retention is governed by the geometry of the tooth preparation, the control of the path of insertion and the ability to provide mechanical keying into surface irregularities. This is not always ideal and lack of retention is a major cause of failure with fixed prostheses. If, in addition, an adhesive bond can be created, this can enhance the retention significantly and resin adhesive technologies have made this possible.
Mechanical properties
It is important that the thin layer of luting agent produced between the tooth and the restoration is able to withstand the large forces that are potentially transmitted through it. In order to resist fracture, a high tensile strength, fracture toughness and fatigue strength are very beneficial in this respect. The situation can be enhanced significantly by ensuring that the restoration produces a good marginal fit, such that a minimal amount of the luting agent is required.
Although only a small amount of the luting agent is exposed at the surface, it is important that the material is able to resist wear. Excessive wear can lead to sub-margination, which, in effect, means that a small groove is formed. Such a groove can become a site for marginal staining and plaque accumulation.
Marginal seal
The luting agent must also provide a good marginal seal in order to prevent recurrent caries. An ideal luting agent should not be susceptible to dissolution in the oral environment so as to maintain the marginal seal. A low solubility in neutral and acid environments is, therefore, important. If the luting agent is able to provide an adhesive bond to the tooth tissues and the restoration, then this will also help to maintain the integrity of the marginal seal. Recent developments in cementation have sought to achieve exactly that but, with the wide variety of materials to be bonded (enamel, dentine, metal, ceramic), a correspondingly wide variety of adhesion promoters have also become available.
Low film thickness
The film thickness is important because a luting agent needs to be sufficiently thin both to fill the space between the crown or bridge and the tooth, and to ensure proper seating of the restoration. A thick film would be unacceptable, as the restoration may end up higher than was originally intended, causing occlusal problems and a need for it to be ground down. Also, a poor marginal fit would result in more cement being exposed at the surface than necessary. As some luting agents are soluble in the oral environment and prone to erosion, this will cause the loss of material at the margin, which can lead to plaque accumulation, staining and recurrent caries.
Ease of use
Many of the luting agents are provided as powder–liquid delivery systems and, as long as great care is exercised to make sure that the correct powder-to-liquid ratio is used on mixing, this should not present a problem. However, there is a tendency to produce a slightly more fluid mix to give rheological properties that allow the luting agent to flow more readily into the space between the tooth and the restoration and produce a very close adaptation. For some materials, changing the powder-to-liquid ratio can have a profound effect on its properties, especially working and setting times, and is therefore not generally recommended.
The working and setting times need to be such that sufficient time is allowed to place the restoration and yet it does not take too long to set once placed. The best way to ensure the correct powder-to-liquid ratio is to follow the instructions for use carefully or to avoid the whole issue by using encapsulated delivery systems.
Radiopacity
It is important for the practitioner to be able to distinguish between a luting agent and recurrent caries under a fixed prosthesis. In order to avoid possible misinterpretation, it is beneficial if the luting agent is more radiopaque than dentine. It also makes it easier to detect possible excess luting agent and marginal overhangs, especially in those difficult-to-see proximal areas.
Aesthetics
Although not a major consideration with metal and metal–ceramic restorations, aesthetics becomes very important when using all-ceramic restoration. For some of the core-reinforced ceramics, a luting agent that has a white/opaque appearance is acceptable, but as the ceramic restoration becomes more translucent, the optical properties become more important. This has meant that, for the highly translucent resin-bonded ceramics, such as those used in the construction of anterior veneers, new luting agents with comparable colour and colour stability, translucency and surface texture have had to be developed.
Choice of luting agent
The oldest luting agent listed in Table 3.8.1 is zinc–phosphate cement, which provides nothing more than a space filler, sometimes referred to as a grout between the restoration and the tooth. Retention depends primarily on the careful design of the tooth preparation and the quality of fit of the restoration, as the cement has no bonding affinity for tooth tissue, metal or ceramic. The newer polyacrylic acid-based cements, such as zinc–polycarboxylate cement and GICs, go a stage further in being able to bond to enamel and dentine and also are claimed to have some affinity for metal and ceramic surfaces. The range of these tooth-adhesive cements has been extended to include the RMGICs. Although these water-based cements have some ability to bond to metals, in general it can be said that these materials do not provide an adequate bond to metal or ceramic restorations for some of the more demanding situations encountered. Hence, new ceramic and metal adhesives would need to be developed for it to impact on prosthetic dentistry to the same degree as new adhesive procedures and materials have changed operative dentistry. It is the advent of resin-bonding technology that most probably has had the biggest impact on the procedures used to retain indirect restorations.
In order to manage this wide diversity of water-based and resin luting agents and associated clinical procedures, for simplicity they will be considered under two categories, namely:
Water-based luting cements
The water-based cements include zinc–phosphate cement, zinc–polycarboxylate cement, GIC and RMGIC.
Zinc–phosphate cements
Zinc–phosphate cement is one of the oldest cements available and continues to be popular because of its long history of clinical success and favourable handling properties. These cements present as a white powder that is mixed with a clear liquid. The powder consists of mainly zinc oxide, with up to 10% magnesium oxide included, and the liquid is an aqueous solution of phosphoric acid of 45–64% concentration.
Presentation
Powder
The powder is fired at a temperature in excess of 1000°C for several hours in order to reduce its reactivity and provide a suitable working and setting time for the cements; the material would set far too rapidly without this firing process.
The magnesium oxide is added, as it helps maintain the white colour of the cement. It has the additional advantages of making the pulverization process of the zinc oxide somewhat easier, and also increases the compressive strength of the cement. Other oxides (such as silica and alumina) have been added in small quantities of up to 5% to improve the mechanical properties of the set material and to provide a variety of shades.
Some formulations include fluorides (usually in the form of a few per cent of stannous fluoride), and are generally recommended for situations where fluoride release is going to be particularly beneficial, such as for the cementation of orthodontic bands.
Liquid
The liquid is buffered with a combination of the oxides that are present in the powder and with aluminium hydroxide, which acts to form phosphates in the liquid. The aluminium is essential to the cement-forming reaction, producing an amorphous zinc–phosphate, while the zinc helps to moderate the reaction, making sure that the cement has the appropriate working time. This control over the working time also helps to ensure that an adequate amount of the powder is incorporated into the liquid.
Setting reaction
When zinc oxide is mixed with an aqueous solution of phosphoric acid, the superficial layer of the zinc oxide is dissolved by the acid. In the case of pure zinc oxide mixed with phosphoric acid, the acid–base reaction first involves the formation of an acid zinc–phosphate:
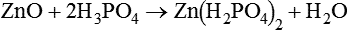
This is followed by a further reaction, where, in this second phase of the process, a hydrated zinc–phosphate is produced:
ZnO+Zn(H2PO4)2+2H2O→Zn3(PO4)2⋅4H2O(hopeite)
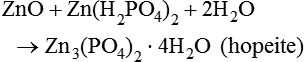
This substance is virtually insoluble, and crystallizes to form a phosphate matrix, which binds together the unreacted parts of the zinc oxide particles. The reaction is slightly exothermic and some shrinkage of the cement takes place.
It is thought that, in the commercial materials, the presence of the aluminium prevents the crystallization process, so producing a glassy matrix in the form of an alumino-phosphate gel. This lack of crystallization is exacerbated by the presence of magnesium, which delays the development of any crystallinity. Some crystallization, resulting in the formation of hopeite, may occur with time.
Unbound water forms globules within the material and makes the cement highly permeable, resulting in a porous structure when the material is dry. The final structure is that of particles of unreacted zinc oxide in a matrix consisting of phosphates of zinc, magnesium and aluminium.
Properties
As a general observation, it is worth noting that zinc–phosphate cements have been around for some considerable time and have provided excellent clinical service. This may be related to the general ease with which the material can be used, as well as the wide range of applications available. They have a well-defined working time and a rapid setting time.
Working and setting times
The working time for most brands of zinc–phosphate cement, when used with the consistency of a luting agent, is usually within the region of 3–6 minutes. The corresponding setting time can vary from 5 to 14 minutes. Both of these times depend on the mixing procedure adopted.
Depending on the application, the material is mixed to either a thick consistency for cavity bases or a thinner consistency when used as a luting agent. The mixing process is carried out by the slow incorporation of the powder into the liquid. The recommended procedure is that, initially, only small increments are added to the powder, followed by a couple of larger increments. Finally, smaller increments are again added, as this will ensure that the desired consistency is not exceeded.
Extended working and setting times can be achieved by mixing the powder into the liquid in increments over a large area of the mixing slab. This helps to dissipate the heat of reaction that would otherwise speed up the setting process. Conversely, the rapid mixing of powder into the liquid will shorten both the working and setting times. This will have the result that a thick mix is obtained, with a low powder-to-liquid ratio, because of the early initiation of the setting process. The low powder content will mean that an inferior material is obtained.
By using a cooled glass slab for the mixing procedure, it is possible to extend the working time without simultaneously increasing the setting time. This also has the benefit of allowing more powder to be added to the liquid, so raising the strength and reducing the solubility. However, great care must be exercised when using this technique, as there is a danger of water contamination either from the slab not having been dried properly or due to condensation. Both will have the effect of reducing the working time. The combination of the cool glass and the incremental process ensures that an adequate working time is maintained. The mixing procedure should be completed within about 60–90 seconds.
The setting time can be extended by a process known as slaking the fluid, in which a small quantity of the powder is added to the liquid about a minute before the main mixing procedure is started.
The consistency of the paste depends on the powder-to-liquid ratio, and it is important that the correct powder-to-liquid ratio is used for the particular application.
For instance, too low a powder-to-liquid ratio would produce a weak and highly soluble material with an unacceptably low pH. Whilst the manufacturers suggest optimum powder-to-liquid ratios for their products, these are difficult to adhere to in practice since the dispensing system is not very accurate. Consequently, most dentists prefer to mix sufficient powder into the liquid until a consistency is obtained which is suitable for the particular application. This makes it all the more important that a consistent and reproducible procedure is adopted.
The liquid is kept in a stoppered bottle. If the top is kept off the bottle, the loss of water by evaporation will lower the pH of the liquid as it becomes more concentrated; this will slow down the setting process. If a lot of water is lost, the phosphoric acid will begin to separate out and the liquid will take on a cloudy appearance. Should this occur, the liquid must be discarded.
When the cement is used as a luting agent, it is important that the powder and liquid are not dispensed until just prior to when they are needed, as evaporation of the water may occur and will slow down the setting reaction. Neither should the material be left for any length of time once mixed because the setting reaction takes place virtually immediately on mixing. If the paste is left for too long, the viscosity will have increased to such an extent that the material will no longer have adequate flow characteristics.
Biocompatibility
A freshly mixed zinc–phosphate cement will have a pH in the region of 1.3–3.6. This low value tends to persist for some considerable time, and it can take up to 24 hours for the cement to return to a near-neutral pH.
When placed over a heavily prepared tooth, the initial pH is sufficiently low to induce an inflammatory response in the pulp. This is especially so if a pulpal micro-exposure is suspected. It is important to remember that the thinner the mix, the lower the pH will be, and the longer it will take for the cement to return to a neutral pH.
Zinc–phosphate cement has no anti-bacterial properties and this, combined with the slight shrinkage on setting, means that it does not provide an ideal barrier to the ingress of bacteria. Thus, the pulpal sensitivity associated with the material may be due to a combination of shrinkage, a lack of anti-bacterial behaviour and the high acidity when freshly mixed, rather than just the high acidity as is generally thought.
The patient may experience some pain during a cementation procedure. This can arise as a result of both the low pH of the cement and the osmotic pressure developed by the movement of fluid through the dentinal tubules. Such an experience is usually only transient and should subside within a few hours. If there is a persistent pulpal irritation, it may have been caused by using too thin a mix of the cement.
The hardening process for a zinc–phosphate cement takes a considerable time, and during the first 24 hours there is a significant release of magnesium with lower amounts of zinc. What biological effects the presence of these various ions might have on the surrounding tissues is not known.
Mechanical properties
As with all other properties, the mechanical properties are very much dependent on the powder-to-liquid ratio of the final cement. The compressive strength can vary from as low as 40 MPa up to 140 MPa. The relationship between the powder-to-liquid ratio and the compressive strength is virtually linear.
The cement shows an initially rapid rise in strength, reaching 50% of its final strength within the first 10 minutes. Thereafter, the strength increases more slowly, reaching its final strength after approximately 24 hours. The cement is extremely brittle, and this is reflected by its very low tensile strength, which is of the order of 5–7 MPa. The modulus of elasticity is approximately 12 GPa, which is similar to that of dentine.
Consistency and film thickness
To ensure the proper seating of the restoration when zinc–phosphate cement is being used as a luting agent, it is important that the cement is capable of forming a very thin film.
On mixing, the powder is partially dissolved in the acid, such that the final size of the remaining powder in the set structure ranges from 2 to 8 µm. As the mix flows readily, a film thickness of less than 25 µm can be achieved. This is adequate for cementation purposes, but the thickness of the layer is very much dependent on the procedure adopted.
The viscosity of the mix increases quite rapidly with time. Within a couple of minutes, the viscosity can already be quite high, although the material itself is still quite manageable. Nevertheless, it is recommended that no undue delay is allowed to occur when cementing a restoration, as the reduced viscosity can result in a significantly higher film thickness for the cement and thus a poorly seated restoration.
Solubility
The solubility of a cement is an important consideration, particularly when it is being used as a luting agent. Dissolution contributes to marginal leakage around the restoration and results in bacterial penetration. This may cause either loosening of the restoration or, what is more likely, the induction of recurrent caries, which may undermine the whole tooth.
The cement is highly soluble in water for the first 24 hours after setting, and the loss of material can range from 0.04 to 3.3%; an acceptable upper limit is 0.2%. After this time, the solubility is much reduced. The solubility is highly dependent on the powder-to-liquid ratio achieved for the cement, with a high ratio being desirable. Once the material has fully set, it remains only slightly soluble in water (with some release of zinc and phosphates), but is still susceptible to acid attack. As the final set takes some time to achieve, it is important that the cement is not unduly exposed to the oral fluids.
The fluoride-containing cements show a continuous release of fluoride over a long period. The fluoride uptake by the surrounding enamel should reduce the likelihood of decalcification, especially when used for the cementation of orthodontic bands.
Applications
The most common application for zinc–phosphate cements is as luting agents for the cementation of metal and metal–ceramic crowns and bridges, although it is also used in other applications such as the cementation of orthodontic bands and as a temporary restoration.
These cements exhibit several advantages in that they:
The easy handling characteristics and their adequate retentive properties have made zinc–phosphate cements highly popular with dental practitioners for over a century.
However, the disadvantages are that they:
These factors contribute to the incidence of recurrent caries associated with cast restorations.
Zinc–polycarboxylate cements
The zinc–polycarboxylate cements were first introduced to dentistry in 1968 when a Manchester dentist had the bright idea of replacing phosphoric acid with one of the new polymeric acids: namely, polyacrylic acid. These materials rapidly became popular with the dental profession, as they provided the first cement that was able to bond to enamel and dentine. The bonding mechanism is the same as that described for the GICs (see Chapter 2.5).
Presentation
These cements come as a white powder and a clear, viscous liquid. The constituents of the powder are zinc oxide and magnesium oxide, and the liquid is a 30–40% aqueous solution of polyacrylic acid.
Powder
The powder is based on the same formulation used for the zinc–phosphate cements, containing zinc oxide with approximately 10% magnesium oxide or, sometimes, tin oxide. In addition, there may be other additives such as silica, alumina or bismuth salts. The powder is fired at a high temperature to control the rate of reaction and is then ground to the appropriate particle size. Some brands also contain stannous fluoride to impart the benefits of fluoride release. Pigments may be present to provide a variety of shades.
Liquid
The liquid is usually a copolymer of polyacrylic acid with other unsaturated carboxylic acids, such as itaconic and maleic acid. (The structures of polyacrylic acid and itaconic acid were presented in Chapter 2.3.) The molecular weight of the copolymer is in the range of 30 000–50 000.
In more recent formulations, the acid is freeze-dried and then added to the powder, in which case the liquid component is distilled water. This method was developed in order to simplify the achievement of the correct ratio between the components, which was difficult beforehand because of the high viscosity of the liquid. The pH is adjusted by the addition of sodium hydroxide, and tartaric acid is added to control the setting reaction.
Setting reaction
The basic setting reaction of these cements involves a reaction between the zinc oxide and the ionized copolymer of acrylic acid and itaconic acid.
On mixing the powder and the liquid, the acid attacks the powder and causes a release of zinc ions. This is followed by the formation of cross-links (in the form of salt bridges), in the same way as occurs for the GICs, except that, in this case, the zinc provides the cross-links rather than calcium and aluminium, as shown in Figure 3.8.1.
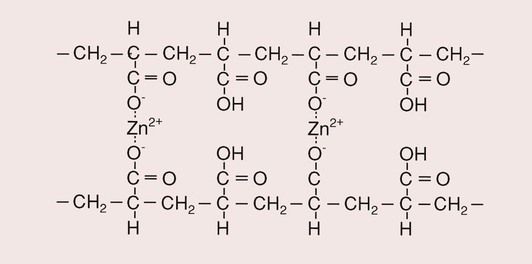
The result of the reaction is a cored structure in which the unreacted powder particles are bound by a matrix of zinc–polyacrylate.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


