Casting alloys for metallic restorations
Introduction
The production of metallic restorations, such as crowns, bridges, inlays, cast posts and cores and partial dentures, in the dental laboratory is carried out by the lost wax casting technique. This method of casting has been around for a considerable time, and is much used by craftsmen to produce intricate jewellery and ornaments. Its history can be traced back beyond 3000 BC, but it was not used in dentistry until the 1890s.
The basic principles are simple. A wax model is produced of the desired shape, and this model is invested in a material resistant to high temperatures. The wax is then removed by melting and burning, leaving behind a cavity of the desired shape. This can now be filled with molten metal, so that the metal assumes the shape of the original wax carving. The stages in the production of a dental casting are therefore as follows:
Thus, it can be seen that many different materials are involved in the production of a metal casting. These include impression materials, model and die materials, waxes, investment materials and casting alloys. Some of these have already been discussed in previous sections.
A detailed account of the various practical stages involved in the production of a metal casting will not be provided here, as this process is the prerogative of the dental technician. Instead, attention will be focused on the alloys that are used and the requirements that are placed on them for their applications in restorative dentistry.
When the lost wax casting technique was first developed by Taggart in the early 1900s, the alloys of choice were gold alloys. For the construction of removable partial dentures, the gold alloys were gradually replaced by cobalt–chromium (Co–Cr) alloys during the 1950s and, to a lesser extent, Co–Cr–Ni alloys. In the latter part of the 20th century, titanium made its appearance as a fixed and removable partial denture-casting alloy.
It is the responsibility of the dentist to request the most suitable alloy for a particular application when instructing a dental laboratory to produce a prosthesis. This choice should not be left to the dental technician. After all, it is the dentist who will be placing these materials in the patient’s mouth.
Desirable properties
The choice of alloy is governed by a number of factors. Cost is a serious consideration due to the increased price of gold today. Other considerations are the biocompatibility of the alloy and its resistance to corrosion and tarnish. It is these factors that particularly limit the range of alloys available for dental applications.
Suitability for a specific application, be it a low-stress-bearing inlay or a posterior bridge, is determined primarily by the mechanical properties of the alloy, such as its stiffness, strength, ductility and hardness. Stiffness is a consequence both of design and of the elastic modulus of the alloy. The higher the elastic modulus, the stiffer the structure will be for the same shape. This is an important consideration, especially for long-span bridges, cast posts, partial dentures and denture clasps. These restorations are also likely to be subjected to fairly high loads and therefore need to be resistant to permanent deformation. This requires the alloy to have a high yield stress or proof stress.
However, for such things as clasps, high strength needs to be balanced against ductility, since it is important that the alloy is not so brittle as to fracture when small adjustments are made.
In the case of inlays, where marginal adaptation is usually improved by burnishing, ductility is even more important. Alloys for these applications need to be very ductile and soft if they are not to fracture during this procedure.
The ease of casting of the alloy is an important consideration for the dental laboratory technician. The dental technician will want to know what the melting range and casting temperature are for the alloy as, in general, the higher these are, the more problems the alloy presents in handling. Another important consideration in this context is the quality of fit of the restoration, which is a function of the casting shrinkage and cooling contraction of the alloy. These have to be accounted for if the casting is not to be too small. The higher the shrinkage, the more of a problem this becomes.
The density of the alloy is also important. Most castings are carried out in a centrifugal force-casting machine, and the higher the density of the alloy, the easier it is to force the air out of the mould space and to fill the space completely with alloy.
Thus, alloys with a wide range of properties are needed to satisfy these varied requirements. The main alloys that are employed in dentistry are noble and precious metal alloys and various base-metal alloys such as Co–Cr alloys and titanium.
Noble and precious metal alloys
The noble and precious metals consist of eight elements that have a number of features in common. They are very resistant to corrosion (noble) and expensive (precious). The noble metals are considered to be made up of gold, platinum, rhodium, ruthenium, iridium and osmium, whereas silver and palladium are generally referred to as the precious metals.
High-gold alloys
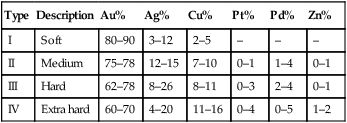
This is a group of alloys that have been around for some considerable time and that can be distinguished from other alloys used in dentistry by their high precious metal content, which must not be less than 75% and a gold content in excess of 60%. The precious metal content is usually made up of gold, silver, platinum and palladium. These alloys can be classified into four distinct groups, as indicated in Table 3.3.1.
Table 3.3.1
Composition of high-gold alloys
| Type | Description | Au% | Ag% | Cu% | Pt% | Pd% | Zn% |
| I | Soft | 80–90 | 3–12 | 2–5 | – | – | – |
| II | Medium | 75–78 | 12–15 | 7–10 | 0–1 | 1–4 | 0–1 |
| III | Hard | 62–78 | 8–26 | 8–11 | 0–3 | 2–4 | 0–1 |
| IV | Extra hard | 60–70 | 4–20 | 11–16 | 0–4 | 0–5 | 1–2 |
The amount of gold in an alloy is defined in one of two ways:
Thus, the dental gold alloys in Table 3.3.1 vary from 21.6 to 14.4 carat, or 900 to 600 fine.
Alloying elements in dental gold alloys
The largest fraction by far of these alloys is gold, with lower amounts of silver and copper. Some formulations also contain very small amounts of platinum, palladium and zinc.
The silver has a slight strengthening effect and counteracts the reddish tint of the copper.
The copper is a very important component, as it increases the strength, particularly of the type III and IV gold alloys, and reduces the melting temperature. The limit to the amount of copper that can be added is 16%, as amounts in excess of this tend to cause the alloy to tarnish.
Platinum and palladium increase both the strength and the melting temperature.
Zinc acts as a scavenger during casting, preventing oxidation, and improves the castability.
A variety of other elements, such as iridium, ruthenium and rhenium (<0.5%), may be present. These have very high melting temperature and act as nucleating sites during solidification, thus helping to produce a fine grain size.
Strengthening mechanism
Although all of the alloying elements give rise to some increase in the yield strength of the gold alloy by forming a solid solution, the most effective strengthening mechanism is the addition of copper, in what is known as order hardening.
This hardening heat treatment is carried out after the homogenizing anneal at approximately 700°C, which is performed to ensure a uniform composition throughout the casting. It involves reheating the alloy to 400°C and holding it at that temperature for approximately 30 minutes. Rather than being randomly distributed, the copper atoms arrange themselves in little ordered clusters.
This ordered structure prevents slippage of the atomic layers, which has the effect of raising the yield stress and the hardness of the alloy. There must be at least 11% copper in the gold alloy for order hardening to occur, so it cannot occur in type I and type II gold alloys. Type III gold alloys have just enough copper, and a small improvement in strength is observed. For type IV gold alloys, the improvement in strength is quite significant.
The effect of this strengthening process is shown in Table 3.3.2. The addition of copper, combined with the hardening heat treatment, can result in a tenfold increase in the yield strength. The importance of the hardening heat treatment for the type III and IV gold alloys is also indicated. However, there is a price to pay in terms of a reduction in the ductility of the alloy, as shown by the lower percentage elongation at which failure occurs. Thus, excessive bending may give rise to brittle fracture, a problem that may arise when producing partial denture clasp arms out of a type IV gold alloy.
Table 3.3.2
Range of mechanical properties of high-gold alloys
| Type | Condition | σy (MPa) | UTS (MPa) | Elongation (%) | VHN |
| I | As cast | 60–140 | 200–310 | 20–35 | 40–70 |
| II | As cast | 140–250 | 310–380 | 20–35 | 70–100 |
| III | As cast | 180–260 | 330–390 | 20–25 | 90–130 |
| Hardened | 280–350 | 410–560 | 6–20 | 115–170 | |
| IV | As cast | 300–390 | 410–520 | 4–25 | 130–160 |
| Hardened | 550–680 | 690–830 | 1–6 | 200–240 |
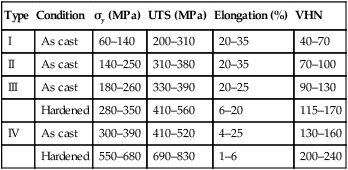
UTS, ultimate tensile strength; VHN, Vickers hardness value.
For some alloys, the hardening process is to allow the alloy to cool slowly on the bench rather than quenching it immediately on casting. This technique is commonly known as self-hardening. The disadvantage with this approach is that it is not as well controlled as when the alloy is first given a homogenizing anneal and then a hardening heat treatment. It is important that the dentist stipulates to the dental technician that a hardening heat treatment is to be carried out if a type III or IV gold alloy is chosen. If a self-hardening alloy has been selected, then it should be allowed to cool slowly on the bench and should not be quenched.
Other features
As the alloying elements form a solid solution readily with the gold, the difference between the liquidus and the solidus is small. This makes these alloys relatively easy to cast and produces a reasonably homogeneous result. The addition of platinum and palladium gives a larger gap between the liquidus and the solidus. The larger this gap, the more compositional segregation occurs on solidification, making a homogenizing anneal more desirable for the type III and IV gold alloys.
Due to their low casting temperature, the casting shrinkage (~1.4%) is readily compensated for by the use of a gypsum-bonded investment.
The low Vickers hardness values (VHN) make these alloys easy to polish to a smooth surface finish, although, in the case of the heat-hardening alloys, this is better done in the as-cast condition.
In general, it can be said that the use of these alloys does not present a major problem to the dental technician, and good-quality, well-fitting castings can be produced. Their corrosion and tarnish resistance is excellent, as is their biocompatibility.
Applications
Given their different mechanical properties, the recommended applications for the use of these alloys is as follows.
Type I alloys
These are best used for single-surface inlays in low-stress situations. As they are relatively soft and easily deformed, they need plenty of support to prevent deformation under occlusal loading. The low yield stress of these alloys allows the margins to be burnished easily. Given the high ductility, they are unlikely to fracture.
Type III alloys
These can be used for all inlays, onlays, full-coverage crowns and short-span bridges, cast posts and cores because of their greater strength compared with type I and type II alloys. However, they will be more difficult to burnish, and have a higher potential for localized fracture if they are burnished excessively.
Type IV alloys
These are used for cast posts and cores, long-span bridges and, in partial denture construction, particularly clasp arms. Clasp arms can be adjusted in the as-cast state and then heat-hardened. Of course, this will not be possible when using a self-hardening alloy. The low elastic modulus and high yield strength of the gold alloy provide a high degree of flexibility to clasp arms, which allows them to be withdrawn over quite severe undercuts without danger of permanent deformation. These alloys cannot be burnished in their hardened state and are therefore unsuitable for inlays.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


