Models, dies and refractories
Introduction
Materials that are derived from gypsum are used in a variety of dental applications. They include:
A model is a replica of the fitting surfaces of the oral cavity; it is poured from an impression of the oral anatomy, and is then used to construct an appliance, such as a full or a partial denture. A mould is used for the construction of a denture. Dies are replicas of individual teeth, and are generally used in the construction of crowns and bridges. A refractory investment is a high-temperature-resistant material that uses gypsum or phosphates as a binder and is used as a mould material for lost wax casting of dental casting alloys and a mould or support structure for the construction of ceramic restorations such as veneers, crowns and inlays using sintering or hot pressing. In this chapter we will focus on the chemistry and properties of these materials.
Models and dies
The basic ingredient for models and dies is gypsum, more commonly known as plaster of Paris.
Chemistry of gypsum
Composition
Gypsum is calcium sulphate dihydrate, CaSO4·2H2O. When this substance is calcined – that is, heated to a temperature sufficiently high to drive off some of the water – it is converted into calcium sulphate hemihydrate (CaSO4)2·H2O, and at higher temperatures the anhydrite is formed as shown below:
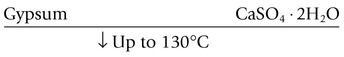
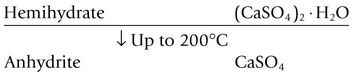
The production of calcium sulphate hemihydrate can be undertaken in one of three ways, producing versions of gypsum with different properties and hence different applications. These are plaster, dental stone and densite (improved stone). It should be noted that the three versions are chemically identical, differing only in form and structural detail.
Plaster
Calcium sulphate dihydrate is heated in an open vessel. Water is driven off, the dihydrate is converted into hemihydrate, known as calcined calcium sulphate or β-hemihydrate. The resultant material consists of large irregular porous particles and these particles do not pack together very tightly. The powder needs to be mixed with a large amount of water to obtain a mix satisfactory for dental use, as much of the water is absorbed into the pores between the particles. The usual mix is 50 mL of water to 100 g of powder.
Dental stone
If the dihydrate is heated in an autoclave, the hemihydrate that is produced consists of small, regular-shaped particles which are relatively non-porous. This autoclaved calcium sulphate is known as α-hemihydrate. Due to the non-porous and regular structure of the particles, they can be packed more tightly together using less water. The mix is 20 mL water to 100 g of powder.
Densite (improved stone)
In the production of this form of calcium sulphate hemihydrate, the dihydrate is boiled in the presence of calcium chloride and magnesium chloride. These two chlorides act as defloculants, helping to separate the individual particles that would otherwise tend to agglomerate. The hemihydrate particles that are produced are yet more compact and smoother than those of the dental stone. The densite is mixed in the ratio of 100 g of powder to 20 mL of water.
Applications
Plaster is used as a general purpose material, mainly for bases and models, as it is cheap and easy to use and shape. The setting expansion (see below) is not of great importance for these applications. A similar composition is used for plaster impression material (see Chapter 2.7) and for gypsum-bonded refractory investments, although for these applications the working and setting times and the setting expansion are carefully controlled by the incorporation of various additives (see below).
The dental stone is used for models of the mouth, while the denser, improved stone is used for individual tooth models, called dies. The latter are used for the shaping of wax patterns from which castings are produced.
Setting process
Heating the hydrate to drive off some of the water produces a substance which is effectively dehydrated. As a consequence of this, the hemihydrate is able to react with water and revert back to calcium sulphate dihydrate as follows:
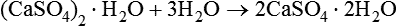
The setting process for gypsum products is believed to occur in the following sequence:
Working and setting times
The material must be mixed and poured before it reaches the end of its working time. The working times vary from product to product, and are chosen to suit the particular application.
For impression plaster, the working time is only 2–3 minutes, whereas it approaches 8 minutes for a gypsum-bonded refractory investment. Short working times give rise to short setting times, as both are controlled by the speed of the reaction. Hence, for an impression plaster, the setting time is typically 2–3 minutes, whereas the setting time can vary from 20–45 minutes for gypsum-bonded refractory investments.
The model materials have working times similar to those of impression plaster but their setting times are somewhat longer. For plaster the setting time is 5–10 minutes, while for stone it can be up to 20 minutes.
The handling characteristics are controlled by the inclusion of various additives. Additives which speed up the setting process are gypsum (<20%), potassium sulphate and sodium chloride (<20%). These act as nucleating sites for the growth of dihydrate crystals. Those that slow down the setting rate are sodium chloride (>20%), potassium citrate and borax, which interfere with dihydrate crystal formation. These additives also affect the dimensional change on setting, as discussed later.
The manipulation of the powder–liquid system will also affect the setting characteristics. The operator can change the powder-to-liquid ratio, and, by adding more water, the setting time is extended, because it takes longer for the solution to become saturated and thus for the dihydrate crystals to begin to precipitate out. Increasing the spatulation time will result in a reduction of the setting time, as it has the effect of breaking up the crystals as they form, hence increasing the number of sites for crystallization.
An increase in temperature has only a minimal effect, since the increased rate of dissolution of the hemihydrate is offset by the higher solubility of the calcium sulphate dihydrate in the water.
Dimensional changes on setting
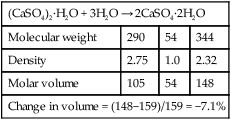
On setting, the crystals that are formed are spherulitic in appearance (Figure 3.1.1), not unlike snowflakes. These crystals impinge on one another as they grow, and try to push each other apart. The result of this action is that there is a dimensional expansion on setting. The material in fact does shrink, in the sense that its molar volume is less by 7.1 vol. %, as shown in Table 3.1.1. However, large, empty spaces form between the crystals, leading to a high porosity. It is this that accounts for the observed dimensional expansion of 0.6 vol. %.
Table 3.1.1
Change in molar volume that occurs as calcium sulphate hemihydrate is rehydrated
| (CaSO4)2·H2O + 3H2O → 2CaSO4·2H2O | |||
| Molecular weight | 290 | 54 | 344 |
| Density | 2.75 | 1.0 | 2.32 |
| Molar volume | 105 | 54 | 148 |
| Change in volume = (148−159)/159 = −7.1% | |||
This ability to expand on setting is a very important feature of this material, and it is the factor that makes it so useful for a large number of dental applications. In particular, models and dies are best produced slightly larger than the oral anatomy. This is to ensure that crowns, bridges and dentures are not too tight a fit when placed in the mouth. The expansion is also made use of in investments, as it helps to/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses