Chapter 27 Dental Implant Surfaces: A Review
The implant design and surface condition influences the dynamics of osteointegration. The following four distinct phases occur in the development of the bone-implant interface:
The overall implant design and the surface condition affect these four processes, often as independent features (Table 27-1). The surgical process of implant dentistry requires initial fixation and lack of relative movement during the initial phases of the development of the implant-bone interface. The implant design is of primary importance to accomplish this initial step; however, the surface condition of the implant may also be a contributing factor. For example, rougher surfaces will aid the implant to have more friction and fixation during insertion of the implant. When the overall design of the implant is a cylinder and/or the bone quality is poor, the surface roughness of the implant will improve the surgical fixation of the implant.
Table 27-1 Studies That Evaluated the Role of Implant Design and Surface Condition on the Bone-Implant Interface
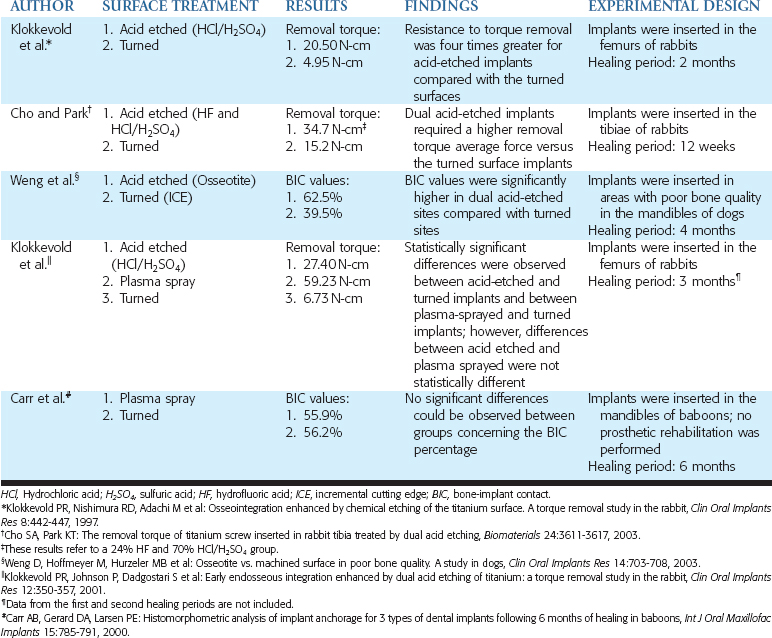
Steigenga et al.1 compared three thread shapes with endosteal implants of similar width, length, thread number, thread depth, and surface condition. The V-shaped and reversed buttress thread had similar BIC and reverse torque values. The square threaded implants had higher BIC and higher reverse torque values. Therefore thread shape may also influence the implant-bone interface during the healing period of an implant, but it is a secondary factor.
The surface condition alone may also decrease the biological width, which causes marginal bone loss when the implant extends through the mucosal tissues. Hermann et al.2 inserted a combination of smooth- and rough-surface implants with smooth collars of 1.5 mm below the bone and other implants with the rough surface placed at the bone crest. Within 1 month the implants with smooth collars lost 1.5 mm of bone, even though the implants received no occlusal load. The implants with a roughened surface to the crest of bone and no occlusal load maintained the bone for the 6 months of the study. Therefore roughened surfaces improve the initial BIC during initial healing and decrease the marginal bone loss when the implant extends through the soft tissue, before occlusal load.
The mature loading period of an implant begins to occur after 3 to 5 years and continues throughout the life span of the implant interface. In general, the surface condition of the implant is least important during this time frame. For example, roughened surfaces on cylinders often lose bone after 5 years of occlusal load. The roughened surface on a cylinder can withstand the initial loads, but fatigue factors of the bone interface begin to break down over continued cycles, similar to shin splints in long-distance runners. The higher the bone turnover rates are because of high strain conditions, the more likely the bone microstrain condition is found to be in the pathologic overload range-and the bone is lost. On the other hand, the implant design is the major feature of an implant body during the mature loading period and is primarily responsible for the bone turnover rates adjacent to the implant. Therefore the implant design becomes most important in the mature loading period.
Before the advent of osteointegration, the use of implant-supported prostheses often resulted in the formation of a fibrous structure around the implants, resulting in mobility and subsequent loss of the implant. The observation that bone can grow over titanium structures started a new pathway in the study of the intraosseous anchorage for dental prostheses.3 As a result, long-term implant success rates are reported to be as high as 90% to 100%.4
Once an implant is inserted in a bone site, a cascade of biological events is initiated. Initially, osteoconduction occurs, which implies the recruitment and migration of osteogenic cells to the implant surface. Secondly, new bone formation takes place, which results in the formation of a mineralized interfacial matrix, followed by a bone-remodeling process. This phenomenon may influenced by the microtopography of the surface.5
However, it is important to emphasize that the surface condition of an implant is not the only requirement to ensure long-lasting implant anchorage. The implant material, status of the bone, surgical technique, surface quality, implant design, and implant-loading conditions all relate to the long-term success of a direct bone-implant interface.6
The percentage of BIC may be used to evaluate the stability of an implant, and values higher than 50% appear to be satisfactory for stable prosthetic results.7 Moreover, torque removal force has been used to describe the anchorage of an implant, in such a way that the higher the value, the greater the biomechanical strength of the bone-implant interface.8 These values are influenced by factors such as the healing period, the loading protocol, and the experimental methodology of the study. It also appears that results obtained in studies performed in humans are more reliable than the findings obtained in studies performed in animals or in vitro. However, it is more prudent to perform some analyses using an animal model (e.g., histologic or torque removal evaluations of successful implants), and in vitro studies are also important to clarify the biological response of cells in contact with different surfaces.
Scientific studies frequently compare the values of one-dimensional parameters to describe the roughness of a surface, based on the evaluation of its peaks and valleys. For example, it is common to use only parameters representative of the height: the arithmetic mean height deviation from a mean plane (also called Ra, if accessed with bidimensional evaluation, or Sa, if accessed with three-dimensional evaluation).9 Each surface should be described by the combination of parameters representative of height and space. To simplify the description and comparison of surfaces found on an implant body, both the values of Ra and Sa are addressed in this chapter.
TITANIUM
The “osseointegration” phenomenon was firstly described by Brånemark et al.,3 and is defined as the “direct contact between living bone and a functionally loaded implant surface without interposed soft tissue at the light microscope level.”10
Titanium is a metal that presents low weight, high strength/weight ratio, low modulus of elasticity, excellent corrosion resistance, excellent biocompatibility, and easy shaping and finishing.11 Because of these properties, it is the material most widely used in the manufacture of dental implants, in the commercially pure titanium (cpTi) form or as an alloy. The most frequently used alloy (titanium-6 aluminum-4 vanadium [Ti6Al4V]) is composed of 90% titanium, 6% aluminum (decreases the specific weight and improves the elastic modulus), and 4% vanadium (decreases thermal conductivity and increases the hardness).12
Lincks et al.13 observed in cell cultures that the osteoblast-like cells responded in a differential manner on plates of cpTi and Ti6Al4V surfaces and speculated that the mosaicism of the alloy components would result in a more complex surface chemistry. Moreover, the authors theorized that ions released from these surfaces would have a negative effect on cell adhesion and could impair normal bone formation.14,15 In addition, they speculated cpTi surfaces were more likely to optimize the osteoblastic differentiation16 and to attach more strongly to bone than to the alloy.17 A study was performed inserting implants in the tibiae of rabbits; results were observed at 1, 6, and 12 months. The removal torque of unloaded implants was not statistically different at 1 month, but at 6 months (29 N-cm and 23 N-cm, respectively, in cpTi and alloy) and 12 months (38 N-cm and 35 N-cm, respectively, in cpTi and alloy) the cpTi implants were significantly more stable. BIC values were not statistically different between the materials, but the authors emphasized a tendency of cpTi implants to have higher values as compared with the alloys implants.18
However, not all studies suggest cpTi improved the BIC. In a report on baboons, cpTi and Ti6Al4V dental implants were inserted in the mandible and maxilla of baboons and analyzed 3 and 6 months after implant placement. BIC values significantly increased over time in cpTi (39.1% to 56.2%) and Ti6Al4V sites (40.0% to 55.2%), but differences at 6 months were not considered statistically significant.19 The primary use of Ti6Al4V in the industry relate to the mechanical advantages over cpTI. The alloy is as much as four times stronger than grade 1 cpTi and 2.4 times stronger than grade 3 titanium. Because the BIC and reverse torque valves are similar before loading, the alloy is often used to decrease the complications of component and/or implant body fracture or wearing of antirotational features of the abutment, which increase the risk of screw loosening.
An interesting aspect of titanium implants (cpTi or Ti6Al4V) is that immediately after its exposure to the air, an oxide layer is formed over the surface (about 2 to 5 nm thickness). This layer is the material that is in contact with the body tissues,20,21 playing an important role in corrosion resistance, biocompatibility, and osseointegration; however, the mechanisms are not completely understood.22–24 This most likely is the reason cpTi and Ti6Al4V have similar BIC values. The titanium oxide layer is mainly composed of titanium dioxide (TiO2),25 and the crystalline structure-the thickness and stability of this layer-varies according to the surfaces of the implant.26–28
TURNED SURFACES
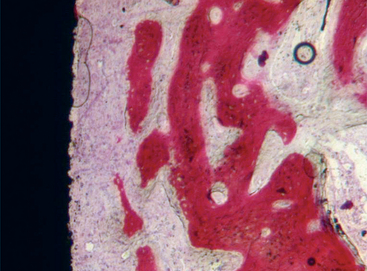
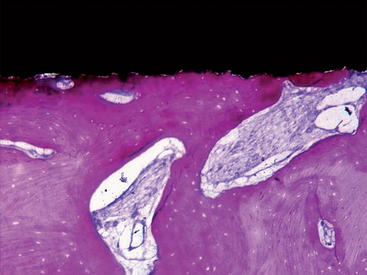
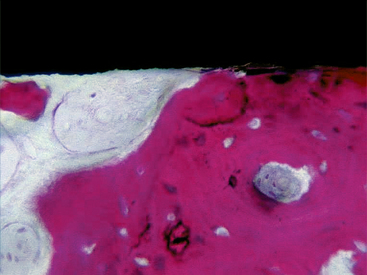
The turned surfaces were the most commonly used surfaces in the past; they were submitted only to a decontamination process after the turning process. These surfaces are also called machined or smooth, but microscopic observation reveals the presence of a slight roughness because of the grooves and ridges produced during the turning process. For this reason, the use of the term smooth should be avoided.12 One of the main characteristics of the turned surfaces is that it is possible to observe a distance osteogenesis (Figure 27-1).
Modifications were proposed to change the characteristics of the surface from turned to roughened, to improve the stabilization of the implant, and to increase the surface area.18,29,30 Moreover, it was observed that the morphology of the surface plays a role in the cellular behavior.31,32 The interaction of the surface with the culturing media and serum seems to directly affect the attachment of osteoblasts and their subsequent proliferation and differentiation,33,34 as well as to influence the production of local cell regulators such as transforming growth factor β (TGF-β) and prostaglandin E2 (PGE2).35,36,37,38
On the preparation of modified surfaces, additive methods (e.g., plasma spraying, HA coating) or subtractive methods (e.g., sandblasting, acid etching) have been used; the optimal type of surface is still to be defined. In one study the ideal roughness for a turned surface, which ideally would result in a stronger bone response, was suggested to be 0.9 to 1.3 μm in average.39 However, another study showed that in the 4.0-μm range, osteoblastic proliferation was reduced but not blocked, phenotypic differentiation was enhanced, and cells on smoother surfaces presented a loss of a differentiated osteoblastic phenotype.13 In fact, the roughness average values seem to be useful only as a reference, and other factors, such as wettability and free energy,38 have been studied to clarify their influences on bone formation, together with the average roughness.
SANDBLASTED SURFACES
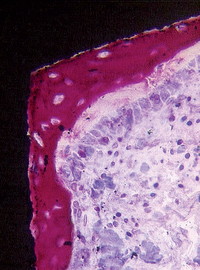
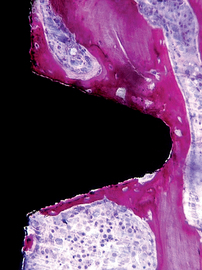
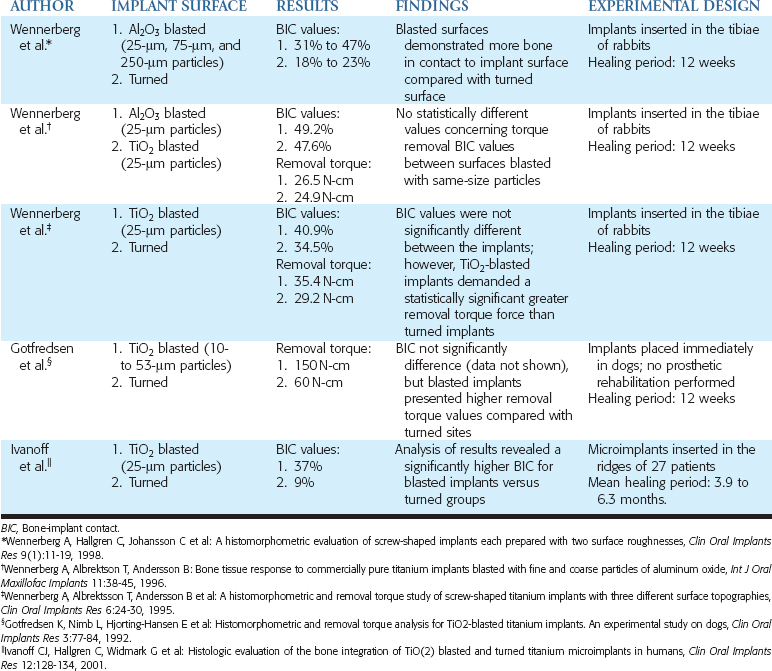
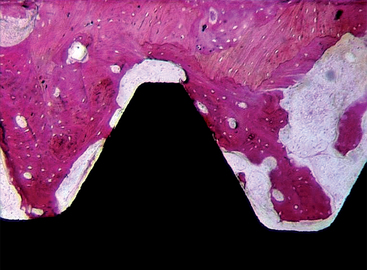
Sandblasting the metal core with gritting agents creates these modified surfaces. This process is influenced by the number and the speed of the rotations to which the implant is submitted, as well as by the pressure and the size of the particles used.12,40 The blasting procedure is performed with the aim of increasing the irregularity of the surface of the implant, using agents such as aluminum oxide (Al2O3, also called alumina) (Figures 27-2 and 27-3) and TiO2. The analyses of different implant surfaces revealed that sandblasted samples showed the largest variability in surface appearance. Researchers have suggested that this characteristic may be explained by an inappropriate refreshing cycle of the used particles because they can break after use; these fragments may be reused, instead of being discarded after a single run.41 In Table 27-2, comparative studies in which sandblasted implants were evaluated are presented.
Sandblasting has been shown in some studies to allow the adhesion, proliferation, and differentiation of osteoblasts.33,42 On the other hand, fibroblasts were found to adhere with more difficulty to this surface; this could limit the soft tissue proliferation and potentially benefit bone formation.43,44
Studies have compared the BIC of turned titanium and sandblasted surfaces with Al2O3 particles. Implants were inserted in the femoral knee joints of rabbits, and the blasted sites presented BIC values statistically higher than the turned surface sites.45 In another study in rabbits, turned implants (Sa = 0.96 μm) were compared with implants blasted with Al2O3 particles of different sizes, to obtain surfaces with different roughnesses. Mean roughness values (Sa) of 1.2 μm, 1.43 μm, and 2.2 μm were observed respectively, with particles of 25 μm, 75 μm, and 250 μm. After 12 weeks, all blasted surfaces (BIC values ranging from 31% to 47%) demonstrated more bone in contact with the implant surface compared with turned surfaces (BIC values ranging from 18% to 23%). Additionally, a higher percentage of bone was found in close contact with the implant surfaces in the sites in which 75-μm particles were used.46
Blasting procedures leave residual particles on the surface of the implant, and this could modify the bonehealing process. Some authors believe that the presence of Al2O3particles remaining may be beneficial to osseointegration, catalyzing this process,47 whereas other researchers believe that aluminum ions may impair bone formation by a possible competitive action to calcium.44,48–50 A study was developed with the aim of describing the effects of residual Al2O3particles on the implant surface on the integration of titanium surfaces. Dental implants were sandblasted (Al2O3 100- to 120-μm particles) or sandblasted and decontaminated (ASTM Committee F [ASTM F] 86-68 decontamination process in an ultrasonic bath), and inserted in rabbits. After 4 weeks, no statistically significant differences were observed between the groups based on the following analyses: BIC value, quantification of multinucleated cells or osteoclasts in contact with the implant surface, quantification of multinucleated cells or osteoclasts 3 mm from the implant surface. The authors suggested these histological results did not provide evidence to support the hypothesis that residual Al2O3 particles on the implant surface could affect the osseointegration of titanium dental implants.51
Alternatives to blasting with Al2O3 particles have also been tested. Blasting a surface with TiO2 particles was proposed to promote a modification on the implant by using a component of the oxide layer naturally formed around titanium implants. In a rabbit comparison study, between surfaces blasted with Al2O325-μm particles (Ra = 0.84 μm) or TiO2 25-μm particles (Ra = 0.96 μm), implants were inserted in the tibia. After 12 weeks, no statistically different values, concerning torque removal (26.5 N-cm and 24.9 N-cm, respectively, in Al2O3 and TiO2 sites) or BIC values (49.2% and 47.6%, respectively, in Al2O3 and TiO2 sites), were observed between these surfaces blasted with the same size of particles.40
Dental implants with TiO2-blasted surface (25-μm particles) (Ra = 0.88 μm) have also been compared with turned implants (Ra = 0.39 μm) in the tibiae of rabbits. Twelve weeks after the insertion, TiO2-blasted implants showed a statistically significant greater removal torque force (35.4 N-cm) than turned implants (29.2 N-cm). However, BIC values were not significantly different between the implants but were generally higher in the blasted group (40.9% and 34.5%, respectively).39 In another animal model, a comparison between TiO2-blasted and turned surfaces was performed by placing dental implants in fresh extraction sockets in dogs. After a 12-week healing period (no prosthetic rehabilitation was performed), no significant differences were observed between the surfaces concerning BIC (69% for both groups); blasted implants, however, showed higher removal torque values (150 N-cm) compared with turned sites (60 N-cm).52
In a comparison study between TiO2-blasted (25-μm particles) (Sa = 1.43 μm) and turned surfaces (Sa = 1.47 μm) in humans, microimplants were inserted in 27 patients and retrieved after a mean healing time of 6.3 months in the maxillae and 3.9 months in the mandible. The analysis of the results revealed a significantly higher BIC for the blasted implants than turned groups (37% and 9%, respectively).53 However, these findings may not necessarily modify a clinical outcome. For example, in a clinical study, implants with TiO2-blasted (Sa values of approximately 1.5 μm) and turned (Sa values of approximately 0.7 μm) surfaces were placed in the edentulous ridges of 18 patients. Prosthetic rehabilitation was performed, and after 2-year follow-up, no statistically significant differences between groups were found in the cumulative success rate (100% and 97.7%, respectively) or in marginal bone loss (−0.2 mm and 0.0 mm, respectively).54
Modifications on zirconium oxide (ZrO2) surfaces have also been proposed to improve the bone-implant interface.55 Implants with modified surfaces were investigated in monkeys. ZrO2implants (Al2O3 sandblasting with 50-μm particles) were compared with titanium (Al2O3 sandblasting with 50-μm particles; followed by hydrogen peroxide [H2O2] and hydrofluoric acid [HF] etching). Nine months after implant placement, metallic prostheses were installed, and 5 months later, BIC values corresponded to 67.4% for ZrO2and 72.9% for the titanium implants. However, differences were not statistically significant.56
PLASMA-SPRAYED SURFACES
The use of implants with plasma-sprayed surfaces has been reported in orthopedics studies since the 1970s.57 Later it was observed that, around dental implants, bone was formed without an intervening layer of connective tissue.58 Plasma-sprayed implants are prepared by spraying molten metal on the titanium base, which results in a surface with irregularly sized and shaped valleys, pores, and crevices,59 increasing the microscopic surface area by 6 to 10 times.12 This topography may improve the fixation of implants by the growth of bone into the coating, forming a mechanical interlock.60
SURFACE COATING
Titanium Plasma Spray
The titanium plasma spray (TPS) surface has been reported to increase the surface area of the bone-implant interface and acts similarly to a three-dimensional surface, which may stimulate adhesion osteogenesis (Figures 27-4 and 27-5). The surface area increase has been reported to be as great as 600%. Although tremendous increase in total surface area occurs at the microscopic level, the actual load-bearing capability of the coating increases the functional area by 25% to 30%, which is still substantial. Porous surfaces in the range of TPS (150 to 400 μm) also increase the tensile strength of the bone-implant interface, resist shear forces, and improve load transfer. The increased surface roughness may also improve the initial fixation of the implant, especially in softer bone. Some evidence indicates that the interface may form faster, but no consensus exists regarding whether that may shorten clinical healing times.
A comparison of the biological response of plasma-sprayed (Ra = 7.345 μm) and turned surfaces (Ra = 0.350 μm) was performed in baboons. Six months after the implantation (no prosthetic rehabilitation was performed), no statistically significant differences could be observed between groups concerning the BIC percentage (55.9% and 56.2%, respectively, in plasma-sprayed and turned surfaces).61 Plasma-sprayed implants were used in studies in rabbits,62 monkeys,63,64 and humans,65–68 even under immediate placement (i.e., implants placed in fresh extraction sites) and immediate-loading conditions.69 In Table 27-3, comparative studies in which plasma-sprayed implants were evaluated are presented.
Plasma-sprayed surfaces obtained with more reactive materials have been proposed to accelerate and enhance the bone ingrowth into pores of the implant surface. An alkali modification was proposed after plasma spraying, using sodium hydroxide solutions at 40° C for 24 hours. The oxide layer in modified surfaces (Ra = 18.2 μm) measured about 150 nm, whereas in plasma-sprayed sites (Ra = 17.6 μm) it measured less than 20 nm. Modified and nonmodified plasma-sprayed implants were placed in the femurs of dogs and analyzed 1, 2, and 3 months after insertion. After the first month, values of the push-out test (2.6 MPa for modified implants, which is about 1.5 times of that of the nonmodified implants) and of BIC (60.5% for alkali-modified implants and 20.2% for nonmodified implants) were significantly higher statistically in modified sites. No differences were observed after the second and third month (values were not presented). The authors suggested that the alkali modification may be beneficial to reduce clinical healing times and thus to improve implant success rates.60
One disadvantage of using the plasma-sprayed implants is the detachment of titanium after implant insertion. Franchi et al.70 investigated the detachment of particles around plasma-sprayed, sandblasted, and acid-etched (Al2O3 with 100-μm particles or ZrO2 with 120-μm particles) and turned implants. Fourteen days after placement in femoral and tibial diaphyses of sheep, titanium debris was observed only in the plasma-sprayed samples. In another investigation in which the tissues around plasma-sprayed and HA-coated implants were evaluated, the presence of granules of titanium was found only around the plasma-sprayed surfaces.71,72 This phenomenon could be related to the friction between the implant surface and the host bone cavity during implant placement, but its implications are not clear.
Acid-Etched Surfaces
Acid-etching a titanium base was proposed to modify the implant surface without leaving the residues found after the sandblasting procedure, to avoid the nonuniform treatment of the surface, and to control the loss of metallic substance from the body of the implant (Figures 27-6 and 27-7).12 This is performed using baths of hydrochloric acid (HCl), sulfuric acid (H2SO4), HF, and nitric acid (HNO3) in different combinations. The roughness before etching, the acid mixture, the bath temperature, and the etching time all affect the acid-etching process. Table 27-4 lists the results from histologic evaluation of retrieved implants from humans.
Researchers compared etched (HCl and H2SO4) and turned surfaces by inserting implants in rabbit femurs.8 The roughened surface was characterized by “an even distribution of very small (1 to 2 μm) peaks and valleys.” The resistance to torque removal was evaluated 2 months after implant placement in rabbits, and it was found to be four times greater for acid-etched implants (20.50 N-cm) compared with the turned ones (4.95 N-cm).
A dual acid-etched technique has been proposed to produce a microtextured (instead of a macrotextured) surface, which could be more predisposed to achieve desirable results.73 This is because higher adhesion of platelet genes and higher expression of extracellular genes were observed in this dual acid-etched surface.74,75 Dental implants were inserted in areas with poor bone quality in dogs (mandibular premolar teeth were extracted and left healing for 8 months). Four months later (no prosthetic rehabilitation was performed), BIC values were significantly higher for dual acid-etched implants (62.9%) as compared with turned sites (39.5%).76
Degidi et al.77 presented the histologic analysis of two dual acid-etched implants (HCl and H2SO4) retrieved from humans. The implants were removed 6 months after implantation because of the positioning of the implants in contact with the inferior alveolar nerve and resultant discomfort. These two integrated implants presented a mean BIC percentage of 61.3%, with no gaps or fibrous tissues present at the interface.
The use of dual acid-etched implants in immediate-loading procedures was histologically investigated in humans. Two implants were inserted in the mandible of a patient to immediately support a provisional fixed partial denture (FPD). Four months after occlusal loading, during the uncovering procedure of the submerged implants, those immediately loaded implants were retrieved, and histomorphometric evaluation revealed high levels of BIC, ranging from 78% to 85%.78 These results were confirmed in an experimental study in humans in which microimplants (measuring 2 μ 5 mm, with one side treated by dual acid etching and the other side left turned) were used. These implants were inserted in the posterior maxillae of 11 patients; 6 months later, the BIC values in dual acid-etched sites (72.35%) were statistically higher than in turned sites (35.32%).79
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses