Obstructive Sleep Apnea
Evaluation and Treatment
Researchers are increasingly finding that a lack of sleep is detrimental for our health. Sleepiness has been linked to increased rates of heart disease, obesity, stroke, and even certain cancers. The exact reasons for these effects are still largely unknown, but they give support to the theory that sleep is the time when our bodies naturally repair themselves on a cellular level.150 Sleep—or the lack of it—is now thought to be a complex process that underpins everything from the ability to learn a new skill to how likely we are to find a novel solution to a problem.243 It is also considered a vital part of happiness and one of the best forms of preventative medicine.288
Sleep disorders include conditions that are characterized by repeated pauses in breathing during sleep, which lead to the fragmentation of sleep and decreases in oxyhemoglobin saturation. The physiologic spectrum of sleep-disordered breathing ranges from partial airway collapse with increased upper airway resistance, which is manifested as loud snoring and episodes of hypopnea, to complete airway collapse and prolonged episodes of apnea. Obstructive sleep apnea (OSA) is clinically defined by frequent episodes of apnea and hypopnea as well as symptoms of functional impairment.13,14,129,130
According to the National Sleep Foundation, some form of snoring or OSA occurs in 90 million Americans.245,246 Approximately 40% of individuals who are more than 40 years old snore, and half of them snore every night. Among habitually loud snorers, the incidence of OSA is at least 17% in men and 15% in women. The Foundation estimates that 16 million of the 20 million Americans with OSA remain undiagnosed. Affected individuals are often aware of snoring, daytime sleepiness, poor sleep patterns, and complaints from bed partners.352 To the educated clinician, the diagnosis of OSA may seem clear, but the affected individuals and their families are often unaware.9,106
Young and colleagues carried out a study to determine the prevalence of sleep-disordered breathing.393–395 With the use of a random sample of 602 employed men and women who were between the ages of 30 to 60 years old and who were studied via overnight polysomnography (PSG), these authors assessed the frequency of episodes of apnea and hypopnea per hour of sleep. The authors determined that this condition affected up to 9% of middle-aged women and 24% of middle-aged men (i.e., an apnea–hypopnea index [AHI] of 5 or more and daytime hypersomnolence).185 Both male and female habitual snorers tended to have a higher AHI of 15 or more.
Rationale for Treatment
The two major rationales for the treatment of OSA are to address 1) pathophysiologic derangements and 2) behavioral derangements.263
The pathophysiologic derangements related to OSA are primarily cardiorespiratory in nature.* They include increased risks of myocardial infarction, stroke, and sudden death.153 Sudden death may be caused by tachycardia and tachyarrhythmias (i.e., atrial fibrillation) as well as by bradyarrhythmias. The documented high prevalence of atherosclerotic coronary artery disease among patients with OSA is possibly related to endothelial damage, with platelet activation and the presence of inflammatory mediators also being involved.153 When OSA is combined with systemic hypertension, an increased incidence of stroke has been reported.138,140,392 A higher-than-average occurrence of idiopathic dilated cardiomyopathy and more frequent episodes of ST-segment depression have also been recognized.118,125,153 The physiologic processes that are believed to be involved in OSA and that predispose patients to these risks include hypoxemia, negative intrathoracic pressure, and the disequilibrium of the autonomic nervous system.42
The behavioral derangements caused by OSA may be the result of excessive daytime sleepiness.161,367 OSA is associated with nocturnal arousals during sleep that occur in response to airway collapse and that result in poor sleep. Excessive daytime sleepiness manifests as sleepiness or fatigue of such a degree that the individual lacks vigilance and does not function as well as he or she should during the day. Other symptoms typically include heavy snoring, known apnea, headaches, difficulty waking in the morning, fatigue, sleepiness after eating, memory loss, having difficultly with work interactions, stressed relationships and sexual performance complaints. There is a documented increased incidence of motor vehicle accidents and job-related injuries among individuals with OSA (e.g., individuals with OSA are 10 times more likely to die in an automobile accident).26,85,103,292 OSA is also known to be a cause of difficulty with concentration at school or during work activities. Over time, the hypoxia that is caused by OSA is believed to result in changes in the neurons of the hippocampus and the right frontal cortex of the brain. Neuroimaging studies have revealed evidence of hippocampal atrophy in people who chronically suffer from OSA. Clinically, it is documented that a greater percentage of patients with OSA have problems with mentally manipulating nonverbal information and with executive processing.100 Interestingly, there is not a direct correlation between these behavioral derangements and the severity of OSA as measured with the AHI or with specific levels of oxygen desaturation. It is important to note that there are multiple causes of excessive daytime sleepiness other than OSA, including volitional sleep deprivation, alcoholism, insomnia, and narcolepsy.
Diagnosis, Characterization, and Assessment of Obstructive Sleep Apnea Severity
Pathophysiology
OSA is characterized by symptoms that result from the recurrent sleep-associated collapse of the pharyngeal airway and that lead to hypoxemia, hypercapnia, and fluctuations in intrathoracic pressure caused by increased respiratory effort, with arousal from sleep required to reestablish airway patency. When awake, protective mechanisms increase the activity of the pharyngeal dilator muscles; however, during sleep, these mechanisms may fail and result in the collapse of the pharyngeal airway.45,73,120,293,329,346,349,387 This is made worse by nighttime horizontal and supine sleep positioning.244,256 Frequent locations of pharyngeal collapse include the velopharynx (behind the soft palate), the oropharynx (behind the tongue), or both.240
Schwab and colleagues have documented an increase in adipose hypertrophy within the floor of the mouth, the soft palate, the pharyngeal fat pads, and the lateral pharyngeal walls in many individuals with OSA.324 In patients with obesity, there is a direct deposition of fat that causes submucosal adipose volume expansion within the pharyngeal walls (thus narrowing the lumen) and the floor of the mouth (thus causing elevation and retro positioning of the tongue), thereby diminishing the actual upper airway breathing space and, in many cases, explaining the greater extraluminal critical closing pressure documented in patients with OSA. Evidence supports the concept that many individuals with OSA have an anatomic predisposition to airway collapse as a result of retrusive jaws and obstructed nasal cavities. An individual’s unique baseline skeletal (i.e., jaw) anatomy may have a dramatic effect on the size (i.e., the luminal diameter) of the upper airway. It is now also appreciated that the variability in upper airway breathing space from one person to the next is influenced by the subject’s developmental or congenital maxillofacial skeletal morphology. Deficient maxillomandibular structural support adds to the environmental causes (i.e., weight gain) that thicken the pharyngeal soft tissues, thereby further narrowing the lumen and diminishing the patency afforded by pharyngeal dilator muscle contraction forces that work to maintain and open the airway. Genetics plays a lesser role in the observed volumes of the tongue, the soft palate, the adenoids, the turbinates, and the tonsillar tissues seen in individuals with OSA. These soft-tissue structures are more likely to be affected by acquired conditions such as weight gain, infections, or allergies. With obesity, there may also be alterations of pharyngeal muscle orientation and function. These factors combine to determine overall upper airway breathing space, pharyngeal muscle function, and, as a consequence, the occurrence of apnea events during sleep.
Schwartz and colleagues measured the critical closing pressures during sleep in individuals with OSA and compared these values to those of controls (i.e., individuals without OSA).327 Individuals with baseline anatomically “large-volume” airways are less dependent on pharyngeal dilator muscles to maintain airway patency than those with intrinsic “small-volume” anatomic airways. Schwartz and colleagues showed that control subjects (i.e., individuals without OSA) typically have critical closing airway values of less than 0 cm of water, whereas those with severe OSA have values of more than 8 cm of water. Multiple diagnostic modalities (e.g., cephalometric x-rays, computed tomography [CT] scans, acoustic reflection, magnetic resonance imaging) confirm that individuals with OSA generally have “smaller-volume” baseline awake airway lumens as compared with controls.264
According to Fogel and colleagues the pharyngeal muscles of primary importance fall into three groups87:
1. The muscles that influence the hyoid bone position (i.e., geniohyoid, sternohyoid)
2. The muscles of the tongue (i.e., genioglossus)
3. The muscles of the soft palate (i.e., tensor palatine and levator palatine)
The activity of these muscles is normally increased during inspiration to stiffen and expand (i.e., open) the upper airway. This counteracts the collapsing influences that would otherwise occur with inspiratory negative airway pressure. During expiration, the muscles normally substantially relax while positive pressure is flowing through the respiratory tree. The tensor palatine is an exception in that it must maintain a constant level of tone throughout the respiratory cycle to preserve a patent airway. Fogel and colleagues stated that the activity of the pharyngeal dilator muscles during wakefulness is tightly controlled to maintain pharyngeal patency.87 In individuals with an anatomically “small-volume” airway, the activation of this negative-pressure reflex (i.e., feedback mechanism) to increase the function of the pharyngeal muscles must occur to prevent or limit apnea. This reflex has been shown to be diminished in apnea-prone individuals for both the genioglossus and the tensor palatine muscles. An individual’s use of specific medications (e.g., alcohol, sedatives, narcotics) will also negatively influence the reflex arc, thereby causing the relaxation of the muscles and further diminishing airway patency.
At least until the age of 65 years, age is positively correlated with OSA severity as demonstrated by an increase in the AHI.152 This increase is substantially greater in the presence of baseline maxillomandibular sagittal deficiency. While a person is awake, neuromuscular mechanisms and body position (i.e., standing or sitting up) can positively influence and compensate for the “small-volume” airway. However, the pharyngeal dilator muscles are less able to effectively contract during sleep, which results in the more frequent collapse of the anatomically small breathing space. The ability of the pharyngeal muscles to compensate for and prevent apnea events becomes progressively more difficult with age and in the presence of weight gain.
Frieberg and colleagues hypothesized that a progressive lesion in the afferent and/or efferent nerve pathways caused by repetitive “snoring trauma” may also contribute to the collapsibility and obstruction of the upper airway that is seen in patients with OSA.89–91 Their study evaluated the hypothesis of the presence of a progressive neurogenic lesion in the pharyngeal muscles in patients with longstanding upper airway obstruction. Biopsies of palatopharyngeal muscle were obtained from patients (n = 21) with habitual snoring and degrees of upper airway obstruction and compared with those of non-snoring control subjects (n = 10). The degree of abnormality found was significantly increased in the study patients as compared with control subjects. The results of their research support the hypothesis of a progressive local neurogenic lesion that is caused by the “trauma of snoring” as a possible contributory factor in upper airway collapsibility.
Sleep electroencephalograms (EEGs) of patients with OSA will differ from those of individuals with upper airway resistance syndrome (UARS) and from those of control subjects.117,128 The arousal response of individuals with OSA is delayed, despite the fact that increased respiratory effort is seen during the obstructive phase. This delayed arousal response has been confirmed by differences in the patterns of EEG findings. Guilleminault and colleagues hypothesized that a difference in sensory input may be the responsible factor for the divergent responses to the abnormal breathing patterns that may exist between individuals with UARS and those with OSA.122 The authors set out to compare the results of a palatal mucosa two-point discrimination response in normal subjects (n = 15), those with OSA (n = 15), and those with UARS (n = 15). The subjects were matched for age, sex, and body mass index. The 45 subjects were submitted to a two-point discrimination study of the palatal mucosa during wakefulness. Individuals with OSA had a clear impairment of their palatal mucosa sensory input with a significant decrement in two-point discrimination. Interestingly, the subjects with UARS and the normal control subjects responded similarly to each other. The responses seen in individuals with UARS indicate that those individuals are more capable of transmitting sensory inputs than individuals with OSA. This may be at least one factor that explains the difference in arousal response that is been documented in patients with UARS as compared with those with OSA.122
The basic anatomic factors that reduce the size of the upper airway include the following:
1. Regional obesity (i.e., increased volume of the lateral pharyngeal fat pads, the soft palate, and the floor of the mouth
2. Baseline (inherent) abnormalities of the maxillomandibular skeletal structures (i.e., the maxilla, the mandible, and the nasal septum)
3. Volumetric enlargement of upper airway soft-tissue visceral structures (e.g., tongue, soft palate, tonsils, adenoids, inferior turbinates) as a result of congenital anomalies, allergies, infections, or tumors.
1. The intranasal region. This portion of the airway extends from and includes the external nasal valves, the internal nasal valves, the turbinates, the nasal septum, and the adenoids.
2. The retropalatal region. This portion of the airway is primarily affected by the position of the maxilla (i.e., the hard palate), the size and position of the soft palate, the tonsils, and the parapharyngeal fat pads.
3. The retroglossal region. This portion of the airway is primarily affected by the position of the mandible, the size and position of the tongue, the fullness of the soft tissues in the floor of the mouth, the parapharyngeal fat pads, and the tonsils.
An increased body mass index (BMI), which is also known as obesity, is a known risk factor for OSA in children, adolescents, and adults (Fig. 26-1).21,88,241,255,337 Peppard and colleagues outlined the cardiovascular risks associated with OSA for weight gain and weight loss.269,268 Excessive body weight was found to be positively associated with OSA,262 and the stability of the individual’s weight was also found to be an important factor. A 10% weight gain predicted a 32% increase in the AHI.319 The same 10% gain in weight also predicted a six-fold increase in the odds of moderate to severe OSA developing. Interestingly, a weight loss of 10% predicted a 26% decrease in the AHI.169 Unfortunately, studies consistently document that weight loss programs in patients with OSA frequently fail miserably. Any weight loss that the individual is able to initially achieve tends to be temporary.167
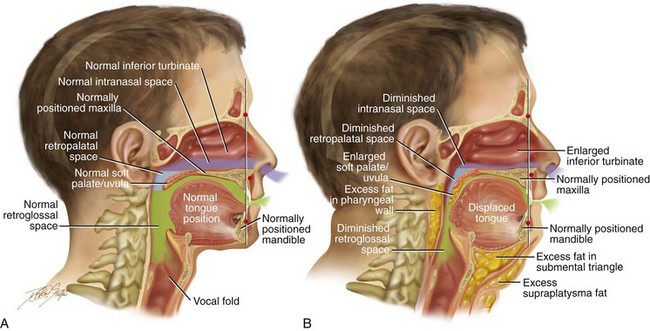
Figure 26-1 A, Midline sagittal cross-section head and neck illustration of a 20-year-old man with a normal body mass index to demonstrate upper airway space and associated maxillofacial anatomy. This individual has a normal upper facial skeleton (cranial vault, orbits, and zygomas), a normal lower facial skeleton (maxilla and mandible), and normal soft tissues (soft palate, tongue, tonsils, and adenoids). The intranasal cavity, which includes the turbinates and the septum, is also normal. B, The same individual when he is 50 years old with an elevated body mass index. The upper and lower facial skeleton remain unchanged with age. The adipose cells, which are scattered throughout and within the upper airway soft tissues, have now increased in volume. This includes the retropharyngeal and the lateral pharyngeal tissues, the soft palate, and the floor of the mouth. As a result, the soft palate is long, thick, and in close proximity to the posterior and lateral pharyngeal walls, thereby diminishing the retropalatal space. As a result of increased adipose volume in the floor of the mouth, the tongue has been displaced superiorly and posteriorly, thus decreasing the retroglossal airway space. As a result of chronic allergies, the patient’s turbinates have enlarged to cause increased nasal airway resistance (i.e., diminished intranasal airway space). There is also excess adipose volume in both the submental triangle and the supraplatysmal locations, thereby resulting in a full neck and a “double chin” appearance. In review, when the patient was 20 years old, he had normal upper airway space, including the intranasal site; the retropalatal site; and the retroglossal sites. When he was 50 years old, the upper airway space is considerably smaller, including the intranasal space, the retropalatal space, and the retroglossal space. The patient depicted here now has documented obstructive sleep apnea.
When an individual’s BMI is elevated, the volume of fat in the pharynx and the floor of the mouth also increases. It is estimated that more than 66% of individuals with OSA are obese. On the basis of the latest World Health Organization statistics, it is estimated that there are more than 1.6 billion overweight adults (from a total population of 6.5 billion) and at least 400 million obese individuals (BMI ≥ 25 kg/m2) on Earth.72 Obesity has both a genetic and an environmental basis.37,63,102,165,237 A review of population studies suggests that heredity accounts for at least 40% of the variance in BMI.219,286 Studies have demonstrated a strong relationship between the BMIs of biologic parents and their children. Interestingly, no relationship between the BMI of adoptive parents and their adopted children has been documented. The best indirect indicator of excessive fat accumulation in the pharyngeal region and the floor of the mouth is a person’s BMI.
Studies have also documented that relatives of individuals with OSA demonstrate a higher incidence of hypoplastic maxillae and mandibles, longer soft palates, and wider uvulas as compared with matched controls.219,326 This also speaks to the hereditary component of a small pharyngeal airway space and to an individual’s genetic predisposition to OSA.295,388
There are specific syndromes and conditions that result in severe maxillomandibular skeleton dysmorphology and that also negatively affect the upper airway (e.g., Crouzon syndrome, Apert syndrome, Treacher Collins syndrome, Pierre Robin sequence).17,38,270,271,287,341,354,390 It is documented that more than 50% of individuals with Down syndrome suffer from OSA.66 Interestingly, a similar percentage of individuals with Down syndrome are known to have a baseline intrinsic developmental jaw deformity that is characterized primarily by maxillary deficiency. The surgical correction of the maxillofacial deformities associated with these syndromes have been documented to enlarge the upper airway space and to improve and often resolve breathing difficulties, including OSA (see Chapters 27, 28, 30 and 31). The surgical management of velopharyngeal insufficiency (i.e., superiorly based pharyngeal flap or sphincteroplasty) in individuals with a repaired cleft palate is also known to result in OSA in some cases.4,39,157,176,187,205,257,258,339,340,343,373,389,396 Interestingly, the non-syndromal developmental dentofacial deformities (e.g., long face growth pattern, short face growth pattern, primary mandibular deficiency, maxillary deficiency with relative mandibular excess, maxillary hypoplasia associated with repaired cleft palate), which affect at least 5% of the general population, also negatively affect the upper airway.212 Unfortunately, these more common patterns of jaw disharmony frequently go unrecognized as significant etiologic factors for OSA (Figs. 26-2 and 26-3). It is incumbent on the otolaryngologist, the pulmonologist or sleep specialist, the pediatrician or internist, the orthognathic or maxillofacial surgeon, and the general dentist or orthodontist to understand the fundamental relationship between developmental jaw deformities and upper airway obstruction in each patient who is evaluated.
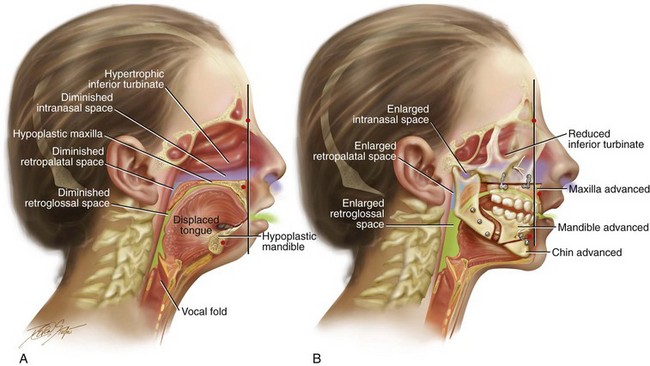
Figure 26-2 Midline sagittal cross-sectional head and neck illustrations of a 16-year-old girl with primary mandibular deficiency, maxillary arch constriction, and Class II excess overjet malocclusion (see Fig. 19-4). The illustrations demonstrate the upper airway space and the associated maxillofacial anatomy before and after jaw and intranasal reconstruction. A, Illustration when the patient was 16 years old, before intervention. The inferior turbinates are hypertrophic as a result of chronic allergies, and the nasal septum is deviated. These findings result in a diminished intranasal airway space. As part of the developmental jaw deformity, the maxilla is retropositioned, and there is a narrow arch width. Therefore, the soft palate is posterior, which results in diminished retropalatal space. The mandible is horizontally (sagittally) deficient, with a posterior displaced tongue. As a result, there is diminished retroglossal space. The tonsils, the adenoids, the tongue, and the soft palate are displaced but otherwise normal. Although the patient is young and has a normal body mass index, the intranasal and jaw deformities result in diminished upper airway space. The patient has difficulty breathing through the nose while she is awake, and is with heavy snoring at night. Obstructive sleep apnea has been documented. B, The patient agreed to a comprehensive orthodontic and surgical approach. Lower bicuspid extractions allowed for the relief of dental compensation. The patient’s surgery included maxillary Le Fort I osteotomy in segments (arch expansion and horizontal advancement); bilateral sagittal split ramus osteotomies (horizontal advancement); osseous genioplasty (horizontal advancement); and septoplasty and inferior turbinate reduction. The postoperative illustration indicates maxillofacial changes that resulted from the reconstruction. Now there is an expanded intranasal, retropalatal, and retroglossal airway space. There is improved nasal breathing during the day and reduced snoring and apnea events at night.
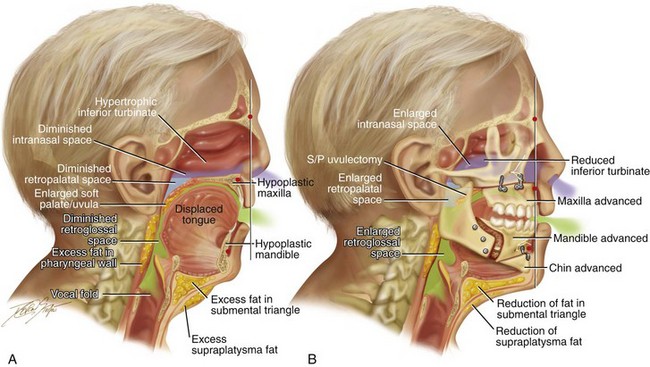
Figure 26-3 Midline sagittal cross-sectional head and neck illustrations of a 59-year-old man to demonstrate upper airway space and associated maxillofacial anatomy before and after jaw, intranasal, and facial surgery carried out primarily to open the airway (see Fig. 26-6). This patient has documented severe obstructive sleep apnea, including a respiratory disturbance index of 51 events/hour with desaturations reaching as low as 82%. The patient has an elevated body mass index and a short face growth pattern that is characterized by maxillomandibular vertical and horizontal deficiency. At the time of presentation to this surgeon, he had already undergone “Phase I” procedures including uvulopalatopharyngoplasty, septoplasty, and inferior turbinate reduction without relief of obstructive sleep apnea. A, Illustration of the patient’s condition before surgery demonstrates hypertrophic inferior turbinates and septal deviation that result in a diminished intranasal space. The maxilla is vertically short and horizontally deficient. The soft palate is long and thick as a result of excess fat accumulation. For all of these reasons, a diminished retropalatal airway space is present. The mandible is horizontally deficient, and the tongue is elevated as a result of excess fat in the floor of the mouth. The lateral and retropharyngeal walls also show excess fat accumulation. These findings account for the diminished retroglossal airway space. B, After the patient was referred to this surgeon, he underwent simultaneous procedures that included maxillary Le Fort I osteotomy (horizontal advancement of 10 mm and vertical lengthening of 5 mm) with interpositional graft; bilateral sagittal split osteotomies of the mandible (horizontal advancement of 12 mm); osseous genioplasty (horizontal advancement); redo septoplasty; and anterior neck soft-tissue procedures (cervical flap elevation, fat removal, and platysma muscle plication). The illustration after maxillary–mandibular–chin advancement demonstrates the enlarged upper airway space, including the intranasal space, the retropalatal space, and the retroglossal space. A 3-month postoperative attended polysomnogram confirms a respiratory disturbance index of less than 5 events/hour.
Not infrequently, the individual with “mandibular prognathism” has undergone a lower jaw set-back treatment to improve the occlusion. Historically, such procedures have been preferred by many clinicians for the treatment of skeletal Class III patterns. However, it is now known that this approach is associated with a risk to the airway size and shape.6,18,41,48,67,68,74,78,84,113,115,171,172,174,314,316,370,386 Demetriades and colleagues confirmed the clinical importance of considering the airway when formulating a treatment plan for the correction of so called “mandibular prognathism.”69 They described 26 patients who presented with developmental dentofacial deformities that were characterized by maxillary deficiencies in combination with relative mandibular excesses. Each patient underwent mandibular ramus osteotomies with at least some set-back. The patients were divided into two groups. The Group 1 patients (n = 13) underwent mandibular setback of at least 5 mm without simultaneous Le Fort I osteotomies. The Group 2 patients (n = 13) also underwent a degree of mandibular set-back, but in conjunction with Le Fort I advancement. All study patients (Groups 1 and 2) underwent preoperative and postoperative lateral cephalometric radiography and portable PSG. The preoperative and postoperative radiographs were compared, and any changes that occurred in the position of the hyoid bone, the distance from the posterior nasal spine to the palate, the retrolingual space, and the hypopharyngeal space were measured. The authors confirmed that mandibular retropositioning of at least 5 mm can significantly decrease the posterior airway space and cause mild to moderate OSA. The study also suggested that, when a limited mandibular set-back is carried out in combination with maxillary Le Fort I advancement, significant negative effects on the airway are likely to be prevented.
In summary, anatomic upper airway risk factors for OSA include the following: 1) regional obesity (i.e., increased upper airway submucosal adipose tissue deposition); 2) retrusive maxillomandibular skeleton (e.g., hypoplasia of the maxilla or mandible); 3) alterations in the intranasal cavity (e.g., septal deviations, hypertrophic inferior turbinates, tight nasal aperture, elevated nasal floor, adenoid hypertrophy); 4) enlargement of other critical upper-airway soft-tissue visceral structures (e.g., soft palate, tonsils, tongue); and 5) inadequate pharyngeal muscular tone (i.e., hereditary and environmental factors).320,332,348,378 Each of these head and neck structural aspects predispose individuals to OSA by causing upper-airway narrowing during wakefulness and airway collapse during sleep. When these aspects occur in combination, the risk is greatly exacerbated. There are also specific medical conditions (e.g., myasthenia gravis, Parkinson disease, amyotrophic lateral sclerosis) as well as certain medications and drugs (e.g. alcohol, narcotics, sedatives, muscle relaxants) that are known to negatively affect the neuromotor tone of the chest wall and the pharyngeal musculature and thus predispose an individual to apnea.
Basic Clinical Evaluation
1. The clinician should determine at what specific maxillofacial skeletal locations (e.g., nasal septum, turbinates, maxilla, mandible), if any, there are negative effects occurring in the upper airway during sleep or while awake.
2. If the patient also has a high BMI (i.e., >25 kg/m2), the clinician should determine at what regional soft-tissue locations (e.g., nasopharyngeal, palatopharyngeal, glossopharyngeal), if any, there is excessive submucosal adipose and other soft-tissue volume that may have a negative impact on the airway.
3. The clinician should determine if there are baseline physiologic dysfunctions of the lungs and chest walls (e.g., chronic obstructive pulmonary disease) that negatively affect the airway.
4. The clinician should consider the effects of baseline neuromotor disorders (e.g., Parkinson disease) and currently used medications (including non-prescription drugs and alcohol) that may negatively affect the airway.
It is also recommended that a subjective questionnaire be used to assess for OSA. The Epworth Sleepiness Scale (ESS) is considered the reference standard.86,163,164 The survey assesses the respondent’s propensity for sleep in eight different situations. Although the survey is not perfect, it does provide useful information about the patient’s waking sleepiness.
An airway analysis that is based on CT scan data may also be a useful tool to assess upper airway space. A recent study used three-dimensional computer analysis from cone-beam CT to present normative upper airway size reference data. The collected data confirm that, in humans, the airway size and length increases until the age of 20 years, at which time there is a variable period of stability. After this stable period, the airway at first decreases slowly in size; then, after the age of 50 years, it decreases more rapidly.322 Li and colleagues demonstrated a correlation between the airway area and the likelihood of OSA.197 There is a high probability of severe OSA with an airway area of less than 52 mm2; an intermediate probability if the airway is between 52 mm2 and 110 mm2; and a low probability if the airway area is more than 110 mm2.190 Cillo and colleagues attempted to correlate specific cephalometric measurements with the AHI in a consecutive series of patients (N = 101) who were documented to have OSA. Unfortunately, no one skeletal or soft-tissue parameter could be linked to OSA.51 Three-dimensional CT airway analysis has documented quantifiable changes in patients’ oropharynges after specific surgical interventions. This type of imaging may also be used to evaluate changes in the airway after specific medical interventions (e.g., weight loss).75
Susarla and colleagues used a case–control study that was designed to analyze the value of the cephalometric upper airway length (UAL) measurement as an indirect predictor of the presence and severity of OSA.360,361 The study group included adults (n = 96) with PSG-documented severe OSA and a control group of adults (n = 56) with skeletal Class II malocclusion without known OSA. Although significant attention has been devoted to describing and measuring upper airway width, little attention has been devoted to measuring UAL. The theory behind this study is that an important component of the airway resistance is the airway length (i.e., the vertical dimension), which is known to be directly correlated with airway resistance per Poiseuille’s law: resistance equals 8 ηL/πr4 (L = length). The results of the study suggest that the UAL (i.e., the distance between the posterior hard palate and the most superior aspect of the hyoid bone on a line parallel to the long access of the airway) is predictive of the presence of OSA in both men and women. The study results indicate that a cephalometric UAL of 72 mm or more in men and 62 mm in women was diagnostic for OSA with high sensitivity and specificity. It would appear that both a narrow posterior airway space and a long UAL predispose individuals to upper airway obstruction and that both conditions are associated with increased odds of OSA.
A reasonable predictor of OSA is a clinical triad of symptoms that includes loud snoring, apnea witnessed by a bed partner, and excessive daytime sleepiness. When the sleep and daytime history is combined with attended PSG, the patient’s clinical head and neck examination (including instrumentation), and a focused radiographic analysis, then an overall picture of the upper airway anatomy and function can be seen.*
Attended Polysomnography
Overnight PSG conducted in a sleep laboratory remains the reference standard for the diagnosis of central sleep apnea or OSA.92,111,155,180,365 PSG requires the use of several monitoring parameters, including EEG, electrooculography, electromyography, electrocardiography, the microphone measurement of snoring, pulse oximetry, respiratory inductance plethysmography, airflow measurement, and the recording of the body position. PSG primarily evaluates sleep-disordered breathing, sleep architecture, and oxygen desaturations. During PSG, episodes of apnea and hypopnea are determined from a reduction in airflow in combination with oxygen desaturation during a 4- to 8-hour time frame.
• Sleep stages (both rapid eye movement and non–rapid eye movement sleep), including the percentage of sleep in each.
• Awake oxygen saturation, including the lowest levels and the stratifications of the percentage of time below specific percentiles (i.e., 90%, 80%, and 70%)
• Heart rate fluctuations (e.g., brachyarrhythmia, tachyarrhythmia)
Characterization of Apnea
Interestingly, it is recognized that 10% to 20% of individuals with known OSA will fail to respond to successful anatomic surgical expansion of the maxillomandibular complex. Another difficult to explain subgroup of individuals with documented OSA is that group with apparent normal upper airway morphology (i.e., no significant narrowing). Complex sleep apnea is a newly recognized category in the spectrum of sleep apnea syndromes that helps to explain these outliers. According to Susarla and colleagues, complex sleep apnea often presents with morphologic features that are typical of obstructive disease but that are combined with PSG evidence of strongly chemoreflex-modulated sleep breathing.360 Complex sleep apnea is distinguished from other forms of OSA by the following:
• Non–rapid eye movement sleep dominance of obstructive disease;
• Oscillations of respiration with or without flow limitation; and
• Induction of central apneas or periodic breathing with the application of positive airway pressure therapy.
Susarla and colleagues believe that a lack of resolution of OSA after successful maxillomandibular advancement surgery may occur if a ventilation control disturbance either remains or is amplified.360 These individuals with chemoreflex-sensitive apnea can be identified, because they are extremely prone to changes in their carbon dioxide levels. It is important to make an accurate diagnosis, because these patients can be expected to require further management of the central component to their apnea. Helpful treatment for this unique subgroup may include the use of nasal oxygen, pharmacologic agents, or positive-pressure–based adaptive ventilation. Affected individuals require continued reassessment and management by a knowledgeable clinical sleep specialist.
Sites of Functional Obstruction
Studies that attempt to localize the site of upper airway obstruction in an individual with moderate to severe OSA have shown that there is rarely a single anatomic site of blockage (see Figs. 26-1 through 26-3). More commonly, multiple sites of upper airway obstruction occur during episodes of hypopnea and apnea. As stated, the potential upper airway sites of obstruction are somewhat artificially grouped as 1) intranasal; 2) retropalatal; and (3) retroglossal. In addition to taking a history and a physical examination, diagnostic studies to further clarify the anatomic levels of obstruction include the Müller maneuver, which is observed during nasoendoscopic instrumentation; lateral cephalometric radiographs taken during end-tidal volume measurement with the Müller maneuver; helical CT; and magnetic resonance imaging.
Intranasal Site
When a history of chronic obstructive nasal breathing is combined with an intranasal clinical examination, instrumentation (e.g., speculum, endoscope), and radiography (e.g., sinus CT scan), the causes of the intranasal obstruction are generally identified. A thickened, buckled, deviated septum (bone and cartilage), enlarged (hypertrophic) inferior turbinates, an elevated nasal floor, and narrow lateral walls (tight nasal aperture) are frequent causes (see Chapter 10). Occasionally, crooked nasal bones, stenotic external nasal valves (after cleft lip repair), or collapsing internal nasal valves (previous nasal surgery) may also be responsible. Enlarged adenoids or nasal polyps may also be causative factors.
Obstructive Sleep Apnea during Childhood: Diagnosis and Management
“The [American Academy of Pediatrics] guidelines contain the following recommendations for the diagnosis of OSA: (1) all children should be screened for snoring; (2) complex high-risk patients should be referred to a specialist; (3) patients with cardiorespiratory failure cannot await elective evaluation; (4) diagnostic evaluation is useful in discriminating between primary snoring and OSA, the reference standard being polysomnography; (5) adenotonsillectomy is the first line of treatment for most children, and continuous positive airway pressure is an option for those who are not candidates for surgery or do not respond to surgery; (6) high-risk patients should be monitored as inpatients postoperatively; and (7) patients should be reevaluated postoperatively to determine whether additional treatment is required.”12,57,328
“This clinical practice guideline is not intended as a sole source of guidance in the evaluation of children with OSA.15,16,35,46,50,108,110,127,170,216,223,235,296 Rather, it is designed to assist primary care clinicians by providing a framework for diagnostic decision making. It is not intended to replace clinical judgment or to establish a protocol for all children with this condition and may not provide the only appropriate approach to this problem.”223
Manifestations in Children
Snoring and difficulty breathing during sleep are the most common complaints reported by parents of children with OSA.54,249,400 However, in contrast with reports of OSA in adults, excessive daytime somnolence is believed to be less common in children, with only 7% of 10% of pediatric patients with OSA experiencing this symptom. Neurocognitive deficits and behavioral manifestations are more frequent in children and may be the result of the chronic exposure to intermittent hypoxemia and sleep disruption caused by sleep fragmentation. Children with OSA are more likely to present with symptoms of hyperactivity, inattentiveness, poor academic performance, and behavioral problems.30,49,60,112,152,166,229,231,253,254,312 Such symptoms are often attributed to attention-deficit/hyperactivity disorder; treatments prescribed for this disorder (e.g., pharmacologic therapy with stimulants) can actually worsen sleep symptoms.
Diagnostic Criteria in Children
Diagnostic criteria for OSA in adults are typically the product of consensus and often include an AHI of 5 or more as documented by nocturnal PSG as well as evidence of disturbed or unrefreshing sleep, daytime sleepiness, or other daytime symptoms.313,382 The rationale for specific AHI diagnostic criteria in children as compared with adults suffers from less available data and heterogeneity across studies. Part of the problem is that few studies have been performed to link specified levels of pediatric OSA with adverse outcomes. At present, an AHI or RDI of as low as 1 event/hour is used to identify children with OSA. Pediatric criteria for scoring AHI or RDI are used until the age of 13 years as a matter of routine. According to the most recent American Sleep Disorder Association guidelines, either the pediatric or adult criteria for OSA may be used for adolescent patients who are between 13 and 18 years old at the discretion of the scoring sleep specialist.12–16 It is also unclear how many events per hour should be considered significant in the adolescent patient as compared with pediatric and adult patients.5
Treatment Considerations in Children
Adenotonsillectomy (T&A) is generally considered to be the standard first-line treatment for a child with OSA who does not have complex medical conditions that would make surgery an unacceptable risk.34,121,124,183,188,220,224,230,232–234,298,317,344,351,355,366,384 A child with OSA should not undergo T&A without receiving a thorough evaluation of the entire upper airway to confirm the actual need for an operation. Nevertheless, the overall cure rate of T&A during childhood is believed to be as high as 82.9%. Studies indicate that, for children with documented OSA and a preoperative RDI of less than 19 events/hour, a T&A will frequently reduce the RDI to less than 5 events/hour. Alternatively, if the preoperative RDI is greater than 19 events/hour, then the T&A has a low probability of reducing the RDI to less than 5 events/hour.355 Other anatomic causes for the child’s OSA should be considered (e.g., maxillomandibular hypoplasia).
A tight nasal inlet is another common cause of upper airway obstruction that may be first recognized during childhood (see Chapter 10). Clinical studies have documented that maxillary arch expansion will improve a tight nasal inlet and thereby decrease nasal airway resistance. Maxillary arch expansion, which is accomplished with the use of a rapid palatal expansion appliance, is commonly carried out during childhood with little morbidity.8,27,36,79,399 Unfortunately, rapid palatal expansion will only address the one site of obstruction and in a limited way. No studies have confirmed that this approach alone will be effective for OSA. For children in whom T&A or rapid palatal expansion is required but does not lead to the resolution of OSA, evaluation to confirm the additional sites of obstruction should follow and appropriate interventions considered. The option of the administration of CPAP should be entertained, but the overall long-term adherence rate for CPAP is unknown and felt to be problematic in this population. Skeletal reconstruction is likely to be beneficial in a child with a craniofacial anomaly that is known to obstruct the upper airway and in whom OSA has been documented.17,38,53,270,271,287,341,354,390 For example, maxillary advancement in a child with either Apert or Crouzon syndrome and documented OSA has proven value (see Chapter 30). When a small lower jaw with a retro positioned tongue (i.e., some children with Treacher Collins Syndrome or Pierre Robin sequence) is the cause of OSA, then mandibular advancement can also be helpful (see Chapter 19). Developmental jaw deformities (i.e., long face growth pattern, short face growth pattern, and isolated mandibular deficiency) occur frequently in the general population, but they often go unrecognized as causes of OSA in children and teenagers (see Chapters 19, 21, and 23). After a developmental jaw deformity has been recognized in a child, OSA should be ruled out.126,383 Referral to an orthognathic surgeon familiar with OSA is recommended if the PSG is positive (Figs. 26-4 and 26-5).
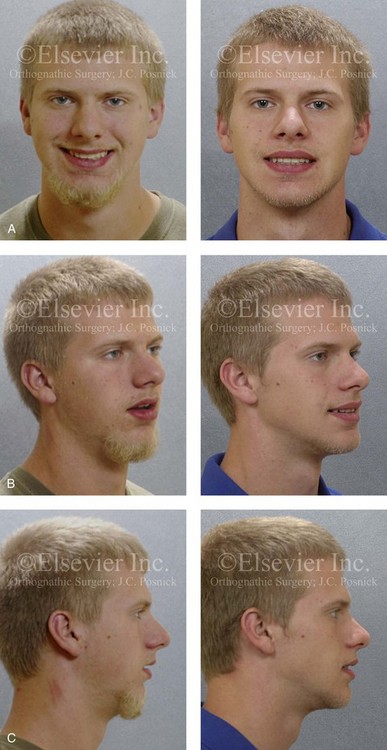
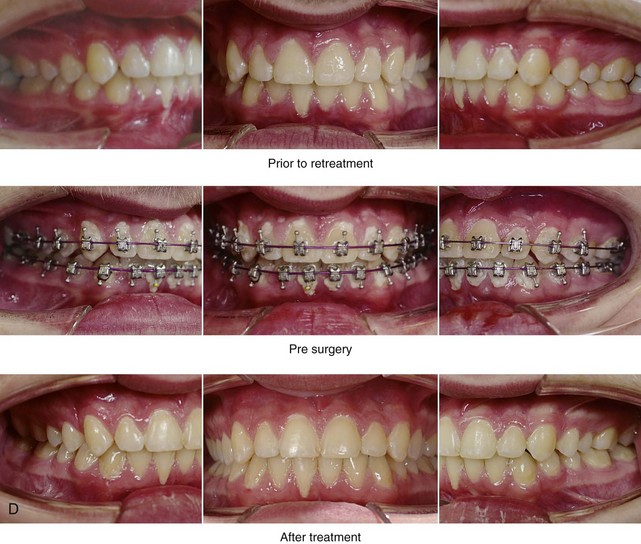
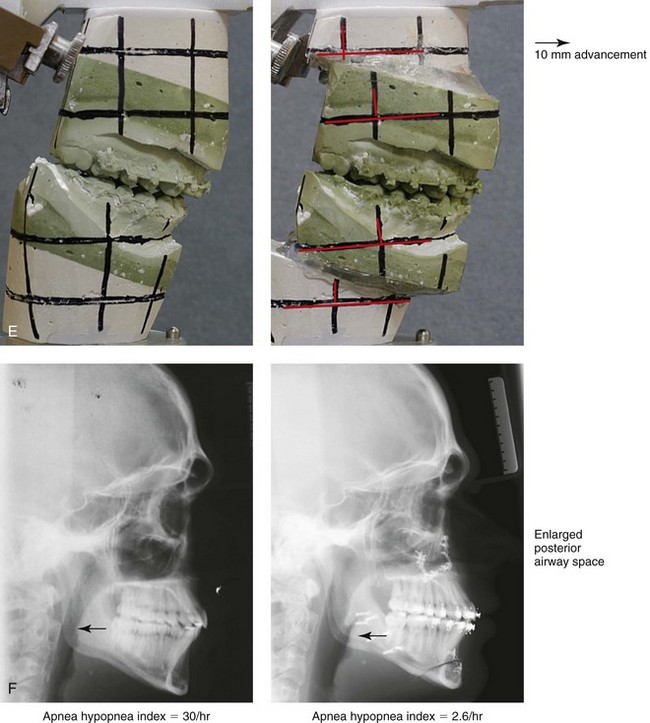
Figure 26-4 A 16-year-old boy was referred by his pediatrician for surgical evaluation. He had a history of heavy snoring, restless sleeping, daytime somnolence, attention-deficit/hyperactivity disorder and other behavioral problems, and poor school grades. An attended polysomnogram documented an apnea–hypopnea index of 30 events/hour. Head and neck examination confirmed a normal-sized and functioning soft palate and tongue. The tonsils and adenoids were not enlarged. The intranasal cavity was obstructed (septal deviation and inferior turbinate hypertrophy). The soft palate was resting close to the posterior pharyngeal wall as a result of maxillary retrusion. The tongue was retropositioned as a result of a retrognathic mandible. The patient had previously undergone 2 years of orthodontics and achieved a Class I canine and molar occlusion. The anterior and posterior facial heights were relatively normal. After consultation with his medical and dental team (general dentist, orthodontist, pediatrician, and sleep specialist), the patient agreed to a surgical approach. Orthodontic appliances were placed to protect the periodontium, and he underwent jaw and intranasal surgery. The patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement +10 mm); bilateral sagittal split ramus osteotomies (horizontal advancement +10 mm); osseous genioplasty (horizontal advancement +10 mm); and septoplasty and inferior turbinate reduction. Three months after surgery, he underwent an attended polysomnogram that confirmed the resolution of the obstructive sleep apnea with an apnea–hypopnea index of 2.6 events/hour. He no longer snored or experienced restless sleep. Daytime fatigue improved, as did his behavioral problems and his schoolwork. A, Frontal views with smile before and after surgery. B, Oblique facial views before and after surgery. C, Profile views before and after surgery. D, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. E, Articulated dental casts indicate analytic model planning. F, Lateral cephalometric radiographs before and after treatment.

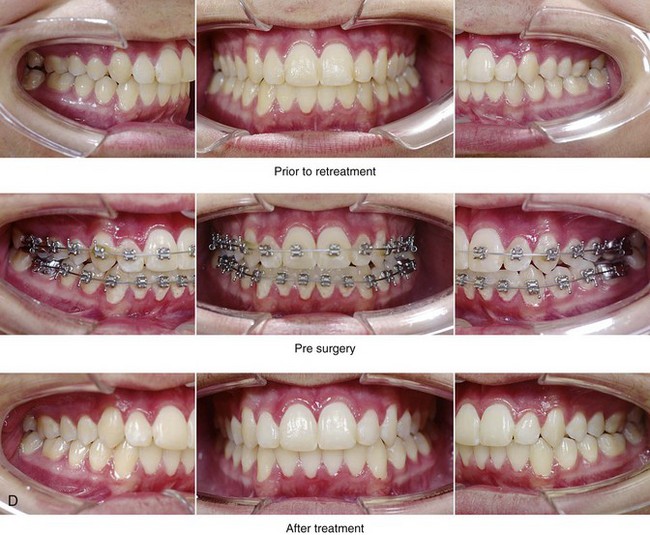
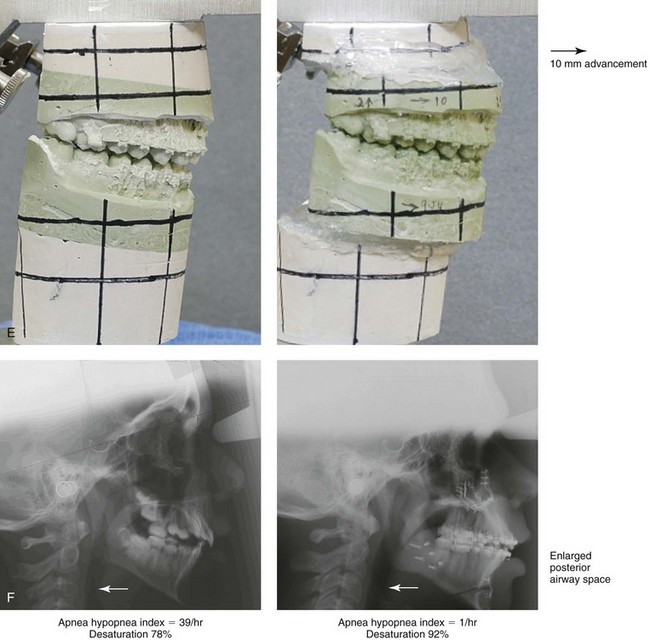
Figure 26-5 A 15-year-old boy was referred by his pediatrician and arrived with his parents for the surgical evaluation of documented obstructive sleep apnea. He had a history of heavy snoring, restless sleeping, and daytime somnolence. He was diagnosed with attention-deficit/hyperactivity disorder when he was 3 years old, and his school grades are poor. An attended polysomnogram confirmed an overall apnea–hypopnea index of 17 events/hour. In the supine position, his apnea–hypopnea index was 39 events/hour with desaturation to 78%. Head and neck examination confirmed a normal-sized and functioning soft palate and tongue. The tonsils and adenoids had previously been removed, and the intranasal cavity was obstructed (septal deviation and inferior turbinate hypertrophy). The soft palate was resting close to the posterior pharyngeal wall, and the tongue was retropositioned. Both the maxilla and the mandible were deficient in horizontal projection, which explained the reduced airway space. The anterior facial height was mildly deficient. The patient previously underwent orthodontics and had achieved a Class I canine and molar occlusion. In consultation with medical and dental specialists (general dentist, orthodontist, pediatrician, and pulmonologist), the patient agreed to a surgical approach. Orthodontic appliances were placed to protect the periodontium, and he underwent jaw and intranasal surgery. The patient’s procedures included maxillary Le Fort I osteotomy (horizontal advancement +10 mm); bilateral sagittal split ramus osteotomies (horizontal advancement +10 mm); osseous genioplasty (horizontal advancement); and septoplasty and inferior turbinate reduction. Three months after surgery, he underwent an attended polysomnogram that confirmed the resolution of the obstructive sleep apnea with an apnea–hypopnea index of 0.9 events/hour and a maximum desaturation of 92%. He was no longer snoring or experiencing restless sleep. His daytime fatigue, behavioral problems, and schoolwork all improved. A, Frontal views with smile before and after surgery. B, Oblique facial views before and after surgery. C, Profile views before and after surgery. D, Occlusal views before retreatment, with orthodontic appliances in place, and after treatment. E, Articulated dental casts indicate analytic model planning. F, Lateral cephalometric radiographs before and after treatment.
Managing the Airway during a Surgical Procedure in the Individual with Obstructive Sleep Apnea
A question has been raised regarding the risk of intubation in a morbidly obese individual with a baseline diagnosis of severe OSA. Neligan and colleagues completed a prospective study to assess whether severity of OSA, neck circumference, and BMI were risk factors for difficult intubation in morbidly obese patients.248 Each study patient (n = 180) underwent preoperative PSG, Mallampati scoring, thyromental distance measurement, mouth opening assessment, and neck circumference measurement. Each study patient was scheduled to undergo a surgical procedure that required orotracheal intubation. A systematic approach to the intubation was developed by the authors that included the placement of the individual into the “ramp” position to facilitate intubation by aligning the three axes: the pharyngeal axis, the laryngeal axis, and the tracheal axis. The ramp position was achieved by placing padding below the individual’s back to elevate the head, upper body, and shoulders above the level of the chest. This was judged by positioning the external auditory meatus at the same horizontal level as the sternal notch. Of the 180 patients enrolled in the study, 124 were documented by PSG to have severe OSA (mean AHI, 31.3 events/hour). The median Mallampati score was 2; the mean neck circumference was 43.9 cm; and the mean Cormack and Lehane grade was 1. The incidence of anticipated difficult laryngoscopy, which was defined as a Cormack and Lehane grade of 3 or 4, was 8.3%. Of the study patients with documented OSA (n = 124), only six required three or more intubation attempts, and only 3.3% were judged as being “difficult to intubate.” A Mallampati score of 3 or 4 predicted difficult intubation, and a mean neck circumference of 43.9 predicted difficulty with laryngoscopy. The study showed no direct relationship between the diagnosis or severity of OSA and the difficulty of laryngoscopy or intubation. There was no relationship between neck circumference or BMI and difficult intubation. The authors believe that their successful management of intubation requirements in this morbidly obese group related to their meticulous approach to airway management and their technique of using the “ramp” position, which aligns the airway axes and facilitates intubation. Some anesthesiologists prefer to use either a GlideScope (Verathon Medical, Bothell, Wash) or an awake fiber-optic nasotracheal laryngoscopy technique when faced with a morbidly obese individual who requires intubation (see Chapter 11).
Treatment Options for Obstructive Sleep Apnea
Recognizing that a significant subgroup of adults who have been diagnosed with OSA have a chronic illness is essential when considering treatment options. In general, obese adults with OSA should be thought of in a similar way to those with diabetes or hypertension. For these individuals, the total elimination of the disease is not generally feasible.63,147,169,344 The goal of any treatment modality in this situation is to improve or control the symptoms and the health risks by reducing the severity. A clear exception to this way of thinking is in the individual with a small-lumen upper airway that is primarily the result of retrognathic jaws. For these individuals, who make up approximately one third of patients with OSA, the correction of the jaw deficiency is expected to be successful for most.29,44,82,144,149,186,197,283,285,311,312
A review of the literature that addresses the management of OSA confirms that a plethora of publications discuss a spectrum of medical and surgical treatment options.181,207 More than a dozen “non-surgical” treatment options are mentioned, including palatal, tongue, and jaw training exercises; controlled sleep positioning; spray medications to reduce soft-tissue edema or to improve muscle tone; the use of a spectrum of oral appliances that have been designed to protrude the lower jaw; and the use of CPAP delivered through a variety of occlusive devices over the mouth and nose. The majority of these treatment recommendations (with the exception of CPAP) are empiric and frequently recommended as “simple” options in an attempt to avoid surgery or the need for CPAP. In addition, more than a dozen surgical options are offered with the intention of affecting either a specific region of the pharynx or a tissue type within the head and neck (e.g., external nasal valves, internal nasal valves, nasal septum, nasal turbinates, soft palate, adenoids, uvula, tonsillar pillars, tonsils, tongue, maxilla, mandible, chin, hyoid bone). Ideally, to ensure compliance with the recommended treatment, 1) the medical or surgical approach should have a biologic basis that is directed at the sites of pathology; 2) a reasonable probability of success should be anticipated; 3) an acceptable level of risk should be clarified; and 4) a realistic level of patient acceptance should be expected.
Bariatric Surgery
Rasheid and colleagues completed a prospective study in a consecutive series of patients (n = 100) that underwent gastric bypass for the management of obesity to determine the effects of the surgery on OSA.290 Before surgery, all patients underwent PSG. The mean preoperative RDI was 40 ± 4. Thirteen patients had no OSA, 29 had mild OSA, and 58 had moderate to severe OSA. At 6 months after surgery, patients’ BMIs had improved (38 ± 1 kg/m2 versus 54 ± 1 kg/m2), and so had their ESS scores (6 ± 1 versus 12 ± 0.1). There were also a significant reductions in minimum desaturations during sleep as well as improvements in sleep efficiency. A shortcoming of the study was its conclusion at just 6 months after surgery.
A study by Pillar and colleagues describes the long-term follow up of patients with regard to both weight loss and upper airway findings after bariatric surgery.273 At 4 months after surgery, there was a significant improvement in the AI from 45 events/hour to 11 events/hour. Over a 7-year time frame, the AI regressed from a mean of 11 events/hour back up to 24 events/hour; however, the AI remained at a much lower level (24 events/hour) than it was before surgery (45 events/hour). In their report, the effectiveness of bariatric surgery was to reduce the mean BMI from 40 kg/m2 (i.e., morbid obesity) to a mean BMI of 35 kg/m2 (i.e., obesity) at 7 years after surgery. During the same time frame, the mean AI was reduced from 45 events/hour down to 24 events/hour. Other researchers have also studied the long-term effects of bariatric surgery on patient health.52,97,116,134
Continuous Positive Airway Pressure Option
The use of CPAP for OSA was first reported in 1981. Since its introduction, it has become the reference standard for non-surgical management.43,65,66,71,105,107,114,132,148,159,160,178,181,217,236,267,356,357 With this method, positive pressure can be continuously delivered through a sealed nasal or mouth mask that the patient wears while sleeping. The positive pressure has the potential to pneumatically splint open the whole pharyngeal airway by preventing the soft palate and the tongue from making a seal with the posterior pharyngeal mucosa (i.e., it acts as a counteracting force to the aforementioned collapsing force in the patient with an anatomic predisposition for airway obstruction). The pressure required to achieve this goal is titrated at a sleep laboratory during PSG. CPAP is a sound, evidence-based treatment option that has been shown to be effective when it is used correctly and consistently throughout the night as well as night after night throughout the patient’s life. If CPAP is used in this way, it will decrease daytime sleepiness and therefore likely improve the patient’s mood and quality of life. The discomfort and difficulty associated with the use of CPAP (e.g., drying of the nasal and oral mucous membranes, dislodgment during sleep, noise, inconvenience when transporting the unit, social displeasure, feelings of claustrophobia) makes long-term compliance a clear problem. Even when defining an acceptable CPAP compliance rate of just 4 hours per night for 70% of nights, only a limited percentage of patients (depending on the study reviewed) who consider themselves users of CPAP are adherent. This does not take into account the significant number of those with OSA who either will not consider trying CPAP or those who have tried it but then refuse to use it. Furthermore, this “acceptable compliance rate” represents only approximately 50% of functional sleep time.77,252,291,318,385 Sleep medicine clinicians continue to appreciate the complexity of the disorder (i.e., complex chemoreceptor apnea and apnea-related behavioral derangements), and they now acknowledge the inability of a majority of patients to stick with CPAP for the long term, even if they do use it for the short term. Nevertheless, all individuals should be advised of the availability of CPAP and encouraged to consider it.
The American Heart Association/American College of Cardiology Foundation released a position statement about the effects of sleep apnea on cardiovascular disease.133 The organizations summarized published studies that confirmed the moderate and variable effects of CPAP therapy for OSA on blood pressure. They concluded that individuals with more severe OSA, difficult-to-control hypertension, and better CPAP compliance are likely to have more substantial blood pressure reduction with CPAP. They also recommended that, for those hypertensive patients who were either not willing to use CPAP or not compliant, other effective methods of managing OSA should be considered (e.g., MMCA).
Oral Appliance Option
The objective of an oral appliance (OA) for the treatment of OSA is to mechanically reposition and hold the mandible in a protruded (forward) location during sleep.259 As the mandible moves forward, so does the tongue. An effective OA must prevent the sealing of the tongue to the posterior pharyngeal mucosa, thereby limiting obstruction at that site.374 The upper airway response to oral appliances varies among individuals. It has been stated that up to 50% of individuals with OSA who use an oral appliance will “respond to some extent.”218 Unfortunately, no matter how technically precise the OA is, it can only affect the retroglossal site.213,325 Most individuals with moderate to severe OSA have obstruction at multiple sites.377 According to published rese/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


