Peri‐Implant Diseases
Brian Kucey1 and Elena Hernandez‐Kucey2
1 Private Practice in Prosthodontics, Edmonton, Alberta, Canada
2 Private Practice in General Dentistry, Edmonton, Alberta, Canada
Introduction
Osseointegration is defined as a direct structural and functional connection between ordered, living bone, and the surface of a load‐carrying implant.1 There has been an exponential increase in the number of implants placed and the number of practitioners using dental implants. At the same time, there has been an extrapolation from full‐arch treatment for the edentulous patient to single and multi‐unit restorations in partially edentulous patients. Concurrently, a change from the initial model of osseointegration involving oral surgeons and prosthodontists to the entire range of providers has evolved. Among the many challenges facing implant dentistry is the goal to maintain and improve treatment outcomes, subjectively and objectively. It is inherent that in the treatment of human beings, despite all efforts, complications will arise. Identification of risk factors to reduce morbidity is paramount.
Executing an optimal plan is essential from the clinician’s perspective, in order to achieve a level of outcome within current expectations. Practitioners agree that patient satisfaction is optimized when we prevent or reduce the incidence of clinical complications, particularly those which result in pain and/or implant loss (osseoseparation). Many quality‐of‐life (QOL) and patient reported outcome measures (PROMs) surveys have been published,2,3 which have provided consumer insight of implant dentistry in addition to the goals of the clinician. Although the patient’s perspective can be significantly different from the clinician’s goals, it is our responsibility to educate the patient on the factors that enhance the treatment outcome, including appropriate and regular maintenance. Optimal health around a dental implant restoration is dependent on a balance between osseointegration and healthy peri‐implant tissues (void of inflammation, bleeding, bacterial pathogens, pain, and suppuration). Peri‐implant diseases are pathologic inflammatory changes that alter this healthy relationship and include reversible and irreversible soft and/or hard tissue changes originating in the marginal peri‐implant sulcus, which can lead to ailing (minor complications), failing, and failed (major complications) implants. Potential risk indicators for peri‐implant diseases include poor oral hygiene, smoking, history of periodontitis, diabetes, genetic traits, alcohol consumption, and implant surface.4 The patient (systemic diseases), dental history (periodontitis), social habits (oral hygiene, smoking, drug abuse), and parafunctional habits are each influential in the changes that arise following osseointegration. Additionally, poor restoration design, residual cement, failing components, and trauma can lead to an untoward result.
This chapter provides insight into the diagnosis and treatment of the spectrum of diseases that afflict soft and hard tissues surrounding previously stable and healthy implants with the goal of helping to provide the clinician with evidence‐based techniques to minimize and address complications.
Peri‐implant diseases
Following confirmation that osseointegration has been achieved, the final restoration has been delivered, and the implant is in function, further remodeling occurs contingent on the patient’s physiologic adaptive capacity. Unfavorable changes to the health of the soft and hard tissues surrounding the implant are defined as peri‐implant diseases. Peri‐implant diseases have been classified as peri‐implant mucositis and peri‐implantitis.5 A less common condition, apical peri‐implantitis, has been excluded from this discussion.
Distinction between natural tooth and implant attachment complex
Gingival tissues and osseous attachments differ between natural teeth and implants (Table 23.1). At the surface of an implant, the connective tissue fibers run parallel to the abutment, circumferentially around the implant to form a gingival cuff. They have a tenuous adhesion relative to the strong attachment of connective tissue fibers on a natural tooth. This results in differences in susceptibility to bacterial invasion and pathways of healing as compared to natural teeth. Natural tooth connective tissue attachment can also limit a disease process laterally, as we observe with a cracked tooth. There is no periodontal ligament or crevicular fluid flow surrounding an osseointegrated implant. The junctional epithelium attachment surrounding an implant is dependent on desmosomes and hemidesmosomes.6
Table 23.1 Natural tooth vs. implant gingival complex and attachment mechanism.
| Healthy natural tooth | Stable osseointegrated implant | |
| Epithelial anatomy | Circular and trans‐septal fibers connected to cementum | Collagen fibers parallel to implant with hemidesmosomes |
| Subepithelial anatomy | Fibroblasts and vessels | More collagen and less fibroblasts/vessels |
| Biological width | 2.04 mm to base of sulcus | 2.04 mm |
| Bone attachment | Lamina dura, periodontal ligament, cementum | Direct contact of osteoid to implant surface |
| Sulcus depth | 0.69 mm plus proximal bone position | 0.69 mm plus implant design and position effect |
| Gingival biotype | Thick or thin | Variable |
| Crevicular fluid | Plasma proteins, desquamating epithelial cells, neutrophilic granulocytes, and monocytes | Same |
| Inflamed gingival crevice | Bacterial toxins, endotoxins, lysosomal proteases, collagenolysis | Same |
| Color of gingival tissues | Coral pink, firmly bound to underlying periosteum of the alveolar bone | Same |
| Keratinized tissue attachment | 2 mm minimum for maintaining periodontal health | Considerable disagreement exists as to how much is necessary around implants |
| Principal attachment mechanism | Sharpey’s fibers from alveolus to cementum | Osteocytes, collagen bundles, glycoprotein layer, titanium oxide |
Optimal health around a dental implant restoration assumes successful osseointegration to bone, healthy soft tissues void of inflammation, bleeding or suppuration, and the presence of adequate keratinized tissue. Inflammatory and noninflammatory peri‐implant diseases alter this healthy relationship and include reversible and nonreversible soft or hard tissue changes originating in the marginal peri‐implant sulcus.
History
The Swedish National Board of Health and Welfare provided a formal assessment of osseointegrated implants in 1975.7 The first widely sourced criteria for implant success was the US NIH (National Institute of Health) Harvard Consensus Development Conference (1978)8 but this did not include osseointegrated implants, as only one center had reported their use (Brånemark). The first published criteria of success for osseointegrated implants was by Albrektsson et al.9 which was based on Brånemark implant data. The five listed criteria lacked detail regarding the peri‐implant soft tissue. One millimeter of vertical bone loss following the first year and then less than 0.2 mm of annual bone loss after the first year of service was the benchmark. A minimum of 85% (5 year) and 80% (10 year) success was proposed for the anterior mandible (zone 1). This was a significant increase in success from the NIH Conference. At the 10th anniversary Toronto Conference (1992)10, Adell11 reported at 15 years the annual bone loss of 0.1 mm after healing and remodeling periods.
Albrektsson and Zarb12 updated the criteria of success and suggested that every implant in a reported study be evaluated as part of a four‐grade scale (success, survival, unaccounted for, failures). The 1986 standard was applied to the posterior mandible and maxillae (zone 2). They proposed zone 1 success be increased to 85% (10 year) and 90% (5 year).
Peri‐implant disease has been reported to be effectively managed with strict recall, even in patients with tobacco use and a history of periodontitis.13 With close recare follow‐ups, marginal bone loss was reported after the first year at 0.9 mm, then 0.05–0.07 mm/year.14–16 With standard treatment follow‐ups, the mean total bone loss was 0.16 mm/year.17
Adell18 summarized the Brånemark experience in four points: implant survival may not be the same as implant success, osseointegration needs a long time to be established (over 12 months), care for the host tissue is paramount, and all requirements for osseointegration to occur must be maintained under control for the long term. Implant recare was also categorized as either one, two, or multiple visits per year.
The references listed above reported the outcomes of machined‐surface external hex implants only. Considering the advent of moderately roughened implant surfaces, the previous criteria for implant success may no longer be acceptable. Numerous factors have been documented to influence marginal bone loss (MBL) surrounding dental implants.19
In addition, lack of a standardized methodology prevents systematic review of the majority of published studies. Some argue that cohort study results concerning MBL might be closer to daily implant practice reality than evidence‐based randomized clinical trials (RCTs).20
A paradigm shift from bone only to prioritize soft tissue concerns
The criteria of success for osseointegrated implants concentrated on rates and extent of radiographic bone loss and marginalized concern with mucositis, as there were little available data regarding the peri‐implant epithelium at the time. The causal relationship between oral microflora (biofilm) and peri‐implant disease as a pathway of implant failure was drawn from periodontal literature. There was weak evidence that plaque accumulations cause inflammation of the peri‐implant mucosa analogous to gingivitis. Bone‐based research by Brånemark and colleagues left questions as to the barrier function of the junctional epithelium surrounding dental implants, particularly when it is compromised during inflammation.21
In spite of the fact that the data were not compelling to construct a periodontal model for peri‐implant disease, similarities have been noted.22 Given the ubiquity of peri‐implant disease, there may have been a rush to judgment to explain the etiology. The prevalence of peri‐implant mucositis has been reported as high as 80% of the subjects and surrounding 50% of the implants. Peri‐implantitis was reported to occur in 28–56% of subjects affecting 12–43% implant sites.23
The term peri‐implantitis describing peri‐implant soft tissue inflammation with subsequent destruction of bone was published in 1965 by Levignac.24 The terms “peri‐implant mucositis” and “peri‐implantitis” were introduced in 1993.25 Although the periodontium and peri‐implant tissues have significant architectural differences, the same bacterial pathogens that afflict the natural dentition seem to be related to peri‐implant diseases. It has been postulated recently that marginal bone loss surrounding oral implants is, in reality, a foreign body response in contrast to a periodontitis‐like disease. Albrektsson et al.26 did admit that following severe marginal bone loss, a secondary bacteria‐mediated infection may follow as a complication to the already established bone loss.
The term “osseoseparation” has been proposed to describe “crestal and interfacial bone–implant interface degradation.”27 There is a philosophical disagreement between the progression of peri‐implant diseases and its relation to the eventual complete failure of osseointegration.
Biofilm
As early as 1978, the medical community stated that chronic infections in patients with implantable devices were caused by biofilms.28,29 Dental plaque is a biofilm which encourages bacterial proliferation on both teeth and implants. The formation of biofilm is multi‐stage, initiating with pellicle attachment to enamel and implants, followed by binding proteins, which promote the adhesion of bacteria and secretions of the extracellular polysaccharides to allow aggregation of bacterial colonies. Mature plaque behaves as a complex microorganism and changes in composition as probing depth increases.30 If the implant surface is exposed, roughness significantly contributes to plaque build‐up. The implant–abutment microgap is also a potential source of microbial contamination affecting the surrounding tissue health. The microbiota colonized in completely edentulous implant patients is different than that in partially edentulous implant patients.31 In patients with a history of periodontal disease, the microbiota on implants is similar to that found in periodontal pockets around teeth, which may indicate a susceptibility to peri‐implant disease.32 Biofilms have strong bonds which require regular effective mechanical and chemical intervention to disrupt.
Peri‐implant mucositis (PIM) and peri‐implantitis (PI)
PIM presents as chronic or acute soft tissue reversible inflammatory changes (redness, swelling, bleeding on probing, increased supracrestal pocket depth) without concomitant loss of supporting bone (Figures 23.1 and 23.2). Suppuration and fistula may or may not be present. There may be mobility of the restoration or restorative components but this does not include mobility of the implant. Pain is limited to soft tissue.
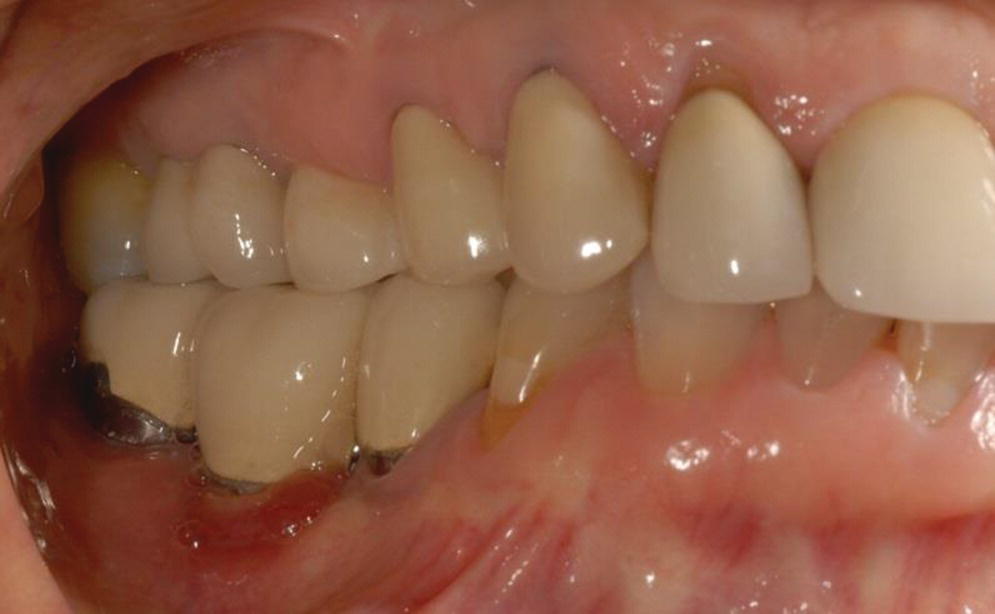
Figure 23.1 Clinical photograph of peri‐implant mucositis lower right molar.
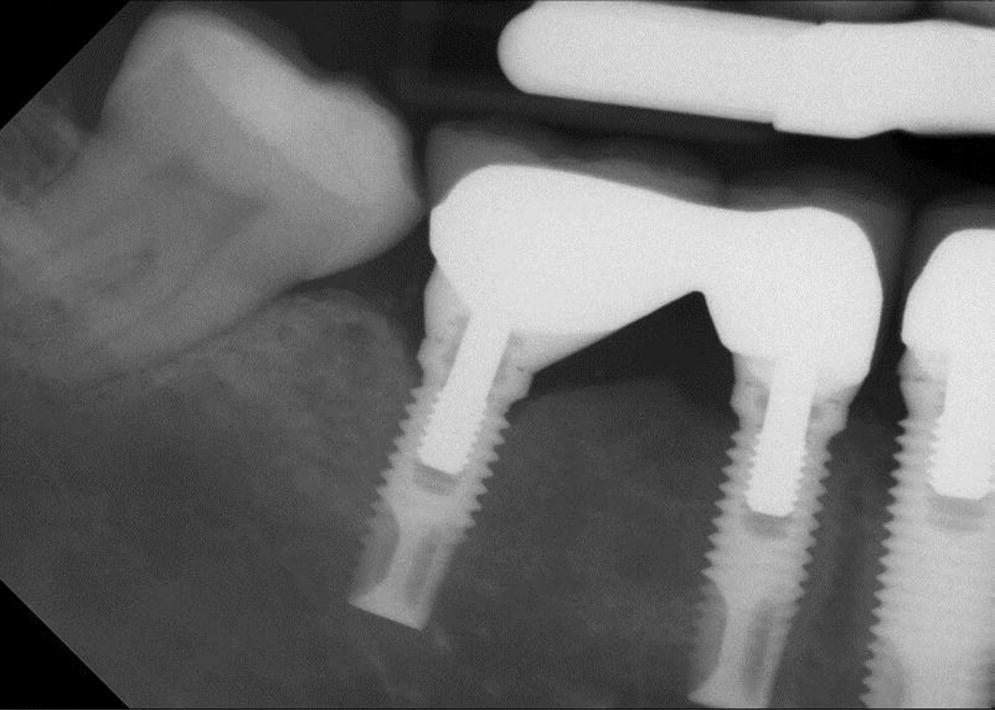
Figure 23.2 Peri‐implant mucositis radiograph lower right first molar.
PI has been defined as site‐specific infectious disease with inflammatory changes in soft tissues and bone around a dental implant and/or its abutment in function (Figures 23.3 and 23.4).33 Most clinicians agree that PI is a “progression” of PIM with an increase in pocket depth (subcrestal changes), bleeding on probing, presence of suppuration (exudate) with or without fistula, possible pain or discomfort, and progressive radiographically determined bone loss.34 The definition disagreements hinge on the presence of suppuration and the percentage of bone loss around the crestal area of the implant. PI is, therefore, a progressive condition following unresolved PIM, with bony defects of increasing dimensions that ultimately could result in complete loss of osseointegration. A proposed subclassification for peri‐implantitis as early, moderate, and advanced has been made.35
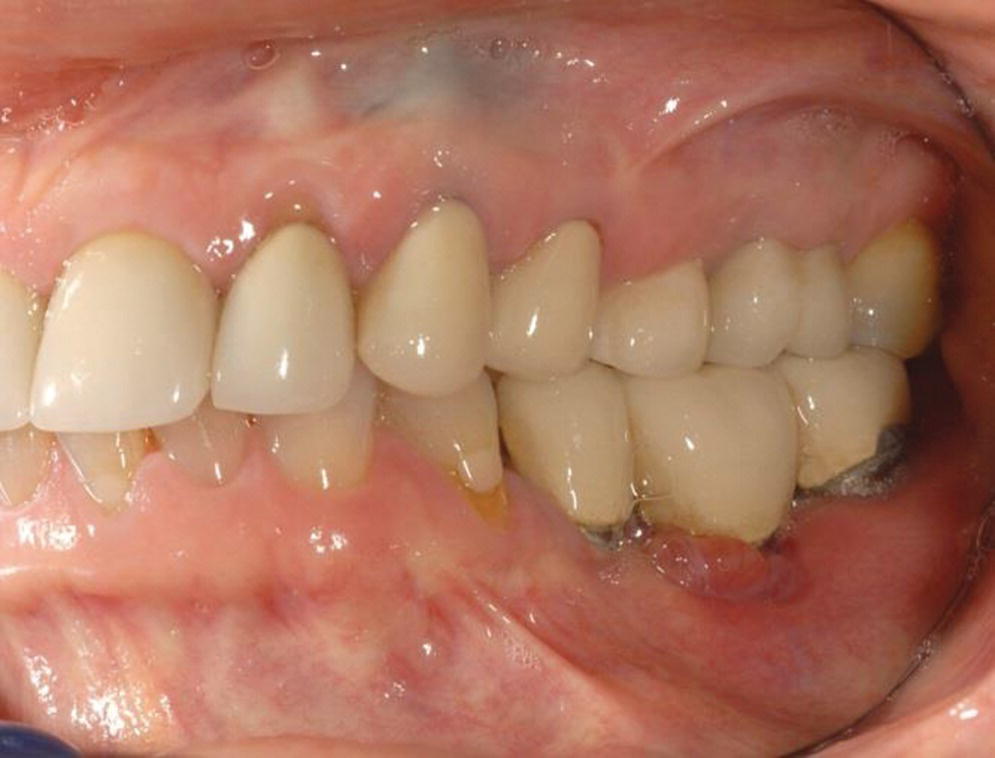
Figure 23.3 Clinical photograph of peri‐implantitis in lower left first molar.
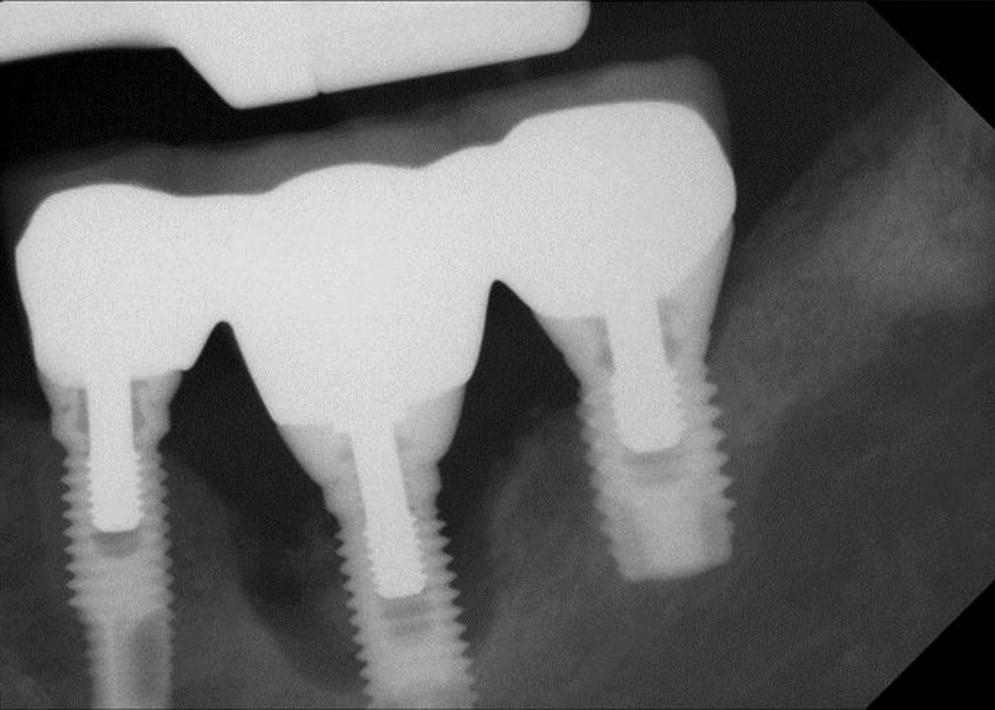
Figure 23.4 Radiograph peri‐implantitis lower left molar.
Both PIM and PI are considered pathologic conditions, often initiated by bacterial insult/origin, and further complicated by trauma, such as occlusal load factors acting in concert,36 implant surface,37 and changes to host resistance related to systemic diseases, medications, and smoking.38 Furthermore, food impaction or foreign object trauma (cement, floss, dental impression materials, oral hygiene aids), with or without plaque around the peri‐implant sulcus, may lead to acute or chronic changes to the peri‐implant soft tissue health.
Diagnosis
A baseline of data related to the original presentation of the individual implant prior to restoration, the implant restoration, soft tissue, bone levels, clinical presentation of abutment and restoration margin, emergence profile, interproximal contacts, occlusion, pre‐load torque, cement type, etc. is necessary to compare findings at subsequent maintenance examinations. Diagnostic tests include accurate updated dental and medical history, evaluation of oral hygiene, probing, serial sequential radiographs, clinical photography, tissue margin changes, torque test, percussion test, bacterial assay,39 floss/shim stock test, articulating paper/shim stock to test occlusion, and test of passive framework fit (Table 23.2). Direct visual inspection of the implant by removal of the prosthesis is recommended when other tests are inconclusive.
Table 23.2 Diagnostic checklist for peri‐implant disease.
| Healthy | Pathologic but potentially reversible | Pathologic and irreversible | |
| Patient concerns | None | Patient awareness | Patient experiencing pain or other symptoms |
| Medical history update | Unchanged | Changes/unrelated | Changes affect implant |
| Evaluation of oral hygiene effectiveness | Good | Fair | Poor |
| Evaluation of tissue health: Loe & Silness Gingival index (0–3) | Mild inflammation: 0 | Moderate inflammation: 1 | Severe Inflammation: 2–3 |
| Probing depth | Minimal (0–2 mm) | 2–4 mm | >4–6 mm |
| Bleeding on probing (BOP) | None | Light to moderate | Heavy |
| Quantity and quality of keratinized tissue | Present and keratinized | Reduced | Reduced/absent with sequelae |
| Gingival recession | None | Slight recession | Advanced recession |
| Trauma, redness, inflammation in gingival tissues | None | Slight, reversible – asymptomatic | Severe, chronic/acute – symptomatic |
| Peri‐implant tissue mobility | None | Mild–moderate mobility | Severe mobility |
| Implant restoration stability | Stable | Suspected movement | Obvious instability |
| Presence of food impaction | None | Occasional | Chronic |
| Interproximal contact | Normal | Weak | Open |
| Residual peri‐implant cement | None | Suspected or asymptomatic | Present and symptomatic |
| Titanium tattoo | None | Suspected | Present |
| Radiographic bone changes | No change | Change within expectations | Change beyond expectations |
| Presence of suppuration or fistula | None | Suspected | Present/recurring |
| Assessment of occlusion | Normal | Heavy contacts without micromotion | Heavy contact with micromotion |
| Presence of pain or discomfort | None | Mild/tissue | Moderate to severe/bone |
| Lymphadenopathy | None | None | If present, acute emergency |
| Mobility of the implant | None | Suspected | Present and irreversible |
| Presence of pathogenic bacteria (assay) | None | Present | Present |
| Passivity of restoration | Passive | Early strain suspected | Non‐passive, rocking or spinning of restoration, cracking of veneering materials |
Radiographic signs
A sign of a peri‐implantitis lesion commonly includes radiographic changes, usually “saucer‐shaped” cratering or vertical changes to the crestal bone, with a normal appearance in the remaining apical portion of the implant. There may be concomitant bleeding and suppuration on probing with inflammation and hyperplasia of the peri‐implant tissues. An associated symptom of pain may be related to an overlying acute infection.
A periapical radiograph 90° to the implant long axis (orthogonal) continues to be the gold standard for assessing subgingival component fit and proximal bone/implant levels. However, it does not provide information regarding bone position on the labial and lingual aspects of the implant. The original Brånemark research team advocated removal of the prostheses annually and two periapical radiographs at 90° to the long axis of the implant, with one of the radiographs with a 5° variation to account for thread pitch. Although it has been suggested that radiographic tube head angulation error within 20° will result in an acceptable radiograph for the external hex implant,40 others have stated that analysis of interface discrepancies becomes subjective when tube angulation error exceeds 5°.41 With the popularity of cemented restorations and numerous internal connection designs, removal of the restoration and/or abutment is not possible or recommended. A paralleling device indexed on the head of the implant and adjacent teeth by occlusal registration material and a conventional film‐holding device (Rinn XCP, Dentsply Rinn, York, PA) at the time of restoration delivery has been advocated. On subsequent radiographic examinations, the paralleling device can be repositioned with the occlusal index without requiring removal of the restoration. An additional observation was, regardless of whether or not the device was applied, the clinicians in the study incorrectly identified misfit up to 30%.42 Preservation and storage of the paralleling device is a logistical concern.
An alternative method to provide predictable radiographic tube positioning is to observe the occlusal screw access restoration for posterior units and to make slight adjustments with tube head if there is a variation from the long axis of the adjacent teeth. For anterior implants, it can be assumed that the angulation of the implant is similar to the long axis of the adjacent teeth, as the majority of these restorations will be cemented. Following the exposure of the digital intraoral radiograph, the tube head and sensor should remain in position relative to the patient for predictable angulation modification, if required. In comparison, film‐based radiographs require not only additional time but increase errors with film replacement, time of developing, and operator and patient movement.
A fistula at implant–restoration level without suppuration may indicate a non‐seated restoration, component misfit (Figure 23.5), trapped debris (compressed particulate graft) between the implant and restoration (Figure 23.6), or thin peri‐implant soft tissue.
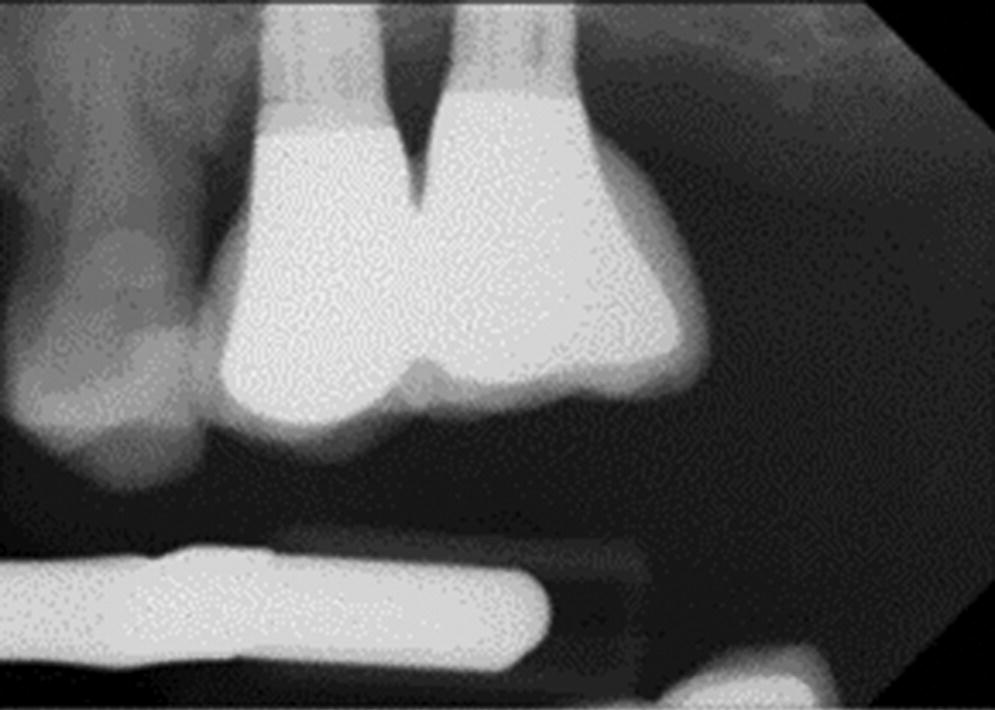
Figure 23.5 Slight abutment misfit on anterior implant with mucositis.

Figure 23.6 Debris identified on inferior surface of abutment.
Percussion tests
Percussion tests using a metal instrument on individual implants, such as a mirror handle, were advocated by the Brånemark research group, with the result similar to that of an ankylosed tooth (sharp sound). A lower tone pitch on an implant may indicate loss of osseointegration. This testing method requires removal of the prosthesis in a complete‐arch restoration and is not quantitative or reproducible. Once the prosthesis is removed, the test will be applied either to the abutment or directly to the implant. A loose abutment screw problem needs to be eliminated prior to testing for implant stability. Percussion testing of single‐tooth implant restorations or short‐span implant bridges may suggest loss of pre‐load torque to prosthetic and/or abutment screws and/or loss of osseointegration without removal of the restoration. This test has been refined with resonance frequency analysis (RFA) instruments (Osstell, Goteborg, Sweden) to determine an “implant stability quotient” (ISQ) which may relate to micromotion of the implant and/or components.43,44 The challenge for single‐tooth implant diagnosis is whether there is movement between the restoration and the implant (i.e. loss of abutment screw pre‐load or decementation), or loss of osseointegration. The use of RFA in relationship to long‐term implant component fatigue has not been established. The mirror handle test remains a simple and accessible clinical diagnostic aid in assessing osseointegration.
Bacteriological testing
Bacteriological testing/microbiological sampling has been advocated in diagnosis of periodontal diseases and dental implants. The BANA (benzoyl‐DL‐arginine‐naphthylamide) test, DNA probes, and immunological reagents are being studied for detection of anaerobic periodontal infections45 and may be useful in the diagnosis of peri‐implant pathogens.46,47
Some investigations regarding the effect of interleukin‐1 gene cluster (IL1A, IL1B) and the IL1RN gene on peri‐implant health have shown a higher risk of peri‐implant diseases in positive‐risk genotype patients.48
Suppuration/exudate
The presence of sulcular exudate with or without a fistula has been discussed in the literature as a significant finding in the transition from peri‐implant mucositis to peri‐implantitis.49 Recent reports show that crevicular fluid tests can now identify progression from health to mucositis to peri‐implantitis.50 Studies analyzing possible markers distinguishing mucositis (with ability to resolve lesion) from implantitis (impaired healing and prolonged inflammation) have suggested tissue plasminogen activators are specific to predict changes in marginal bone. Clinical presentation may include inflammation and redness, with or without discharge.
Occlusion
Use of shim stock and articulating film/paper/ribbon/tape for axial and nonaxial load analysis is a commonly utilized test for both natural teeth and implant restorations. An electronic device has been proposed for diagnosis of occlusion but has a lack of evidence to support its use. Assessment of opposing natural teeth in fremitus, testing for screw loosening, evaluating veneering materials fracture,51 and identifying wear faceting of restorations, all may be important disclosers of overload.
At the time of implant restoration delivery, it is well accepted for the partially edentulous patient there should be no contact with the opposing dentition using shim stock. If using some type of articulating film, there should also be lighter contact on the implant restorations compared with natural teeth. The rationale for this theorem is extended from the fact that a periodontal ligament is absent in osseointegration. A debate continues as to how much occlusal relief is appropriate.
During implant recare, the occlusal contacts on restorations should be evaluated in a similar fashion. A significant finding is a change in the occlusal relief (shim stock) and the size of the contact(s) (articulating film). The proximal contacts should also be evaluated with shim stock and dental floss or tape. These findings are correlated with the radiographic findings. In cases where radiographic changes are not present, the occlusion may be left unaltered.
Occlusal overload has not been demonstrated to directly cause osseoseparation. However, it has been implicated in mechanical failure of screw‐joint stability, and tensile strain, which may progress to implant fracture. On multiple implant restorations, determination of implant fracture may require removal of the restoration for diagnosis.
Parafunctional loading has been suspected as an etiological factor in mechanical failure of implants. Prescription of occlusal splint therapy has been suggested as a preventative measure in these patients.52 However, there are no studies which clearly establish a relationship between bruxism, implant failure, and lack of occlusal splint wear. Occlusal splint wear continues to be recommended in selected bruxers based on expert opinion.53,54
Etiology
Although there is disagreement that all implant systems experience some bone loss after the first year post‐surgically, occasionally some implants demonstrate much greater changes. Currently, there are several theories regarding causes of peri‐implant disease, including bacterial infections, occlusal overload, trauma, incongruent restoration design, poor surgical placement or restoration, and host variables which can contribute to progressive bone loss, including changes in host resistance (immunosuppression or epigenetics), drug interactions (bisphosphonates, antidepressants), nutritional deficiencies, systemic diseases (diabetes), and smoking.55 The etiology is subject to the health of the host and peri‐implant tissue, implant design, presence of roughness, and excessive mechanical load. Misch summarized the etiology of the most common implant complications in his stress‐based treatment plan theorem.56
Bacterial infections
Biologic causes of inflammatory peri‐implant disease are related to the complex biofilm harboring numerous known microbes. Biofilm formation around implant surfaces occurs as early as 30 minutes after the implant is exposed to the oral cavity.57 Osseointegration of the dental implant is influenced by surface roughness but greater surface roughness is directly linked to higher rate of biofilm formation surrounding the implant.58 Apical migration of pathogenic bacteria with subsequent peri‐implant soft tissue inflammation and progressive bone changes are thought to be more aggressive processes than sequelae of bacteria around natural teeth in periodontal disease.59 Microflora surrounding implants with peri‐implantitis have increased red and orange complex species as defined by Socransky.60 The red complex species include Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola, and the orange complex species include Fusobacterium nucleatum and Prevotella intermedia. A fungal component, Candida albicans, has also been identified in bacterial assays and found to have increased adhesion to titanium implant surfaces.61 Microbiota of the remaining natural teeth, particularly in the partially edentulous patient, serve as the primary source of causative pathogens and directly affect implants undergoing osseointegration.62 Demonstrated susceptibility to periodontal disease appears to translate to increased susceptibility to peri‐implantitis,63,64 although patients under periodontal care and strict recare have been reported to have similarly high implant success.13
Mechanical factors
Mechanical causes of peri‐implant disease include instability of the restoration, associated with loss of screw pre‐load,65–67 failure of cement retention of restoration,68,69 failure to remove excess cement (Figures 23.7 and 23.8),70,71 excessive contour of restoration,72 prosthesis misfit,73,74 and excessive depth of manufacturer implant–abutment interface,75 hindering oral hygiene access and promoting pathogenic biofilm. Unfortunately, a lack of agreement on study design has left the profession with a conclusion that there is a lack of evidence to claim superiority for a single implant design or manufacturer. Touati76 subdivided mechanical factors into implant related (design, surface, type of connection, and platform shifting) and prosthetic related (type of abutment connection, final abutment design, immediate provisionalization, interim abutment material and design, submergence profile, emergence profile, restoration anatomy, retention, possible excess cement, occlusion/excessive load). Lack of precision or passive fit between adjacent implant abutments can result in abutment and/or restoration loosening or fracturing, fracture of the implant, or interruption of osseointegration.77 Impaction of foreign materials, including food particles, popcorn hulls, floss, and oral hygiene aids (brush bristles), can also cause mechanical irritation of the peri‐implant tissues and this is not visible on radiographs.78
Figure 23.7 Clinical photograph of upper left central incisor implant with peri‐implant mucositis due to residual cement.

Figure 23.8 Clinical photograph of recovered residual cement fragment.
Occlusal overloading
Osseointegration changed the thinking again regarding occlusion. Several animal studies have indicated that occlusion is not a causative factor in loss of osseointegration. However, there is a lack of “shock absorption” due to the absence of a periodontal ligament around a dental implant. This means load is transmitted directly to the implant interface with the surrounding bone which, when excessive, can result in microfractures or loss of osseointegration, possibly by facilitating bacterial penetration. Crestal loads have been identified as the most concentrated, which incidentally is where initial bone change occurs. There is a wide variation of this sign of osseous change within implant designs. Lateral occlusal forces are considered more detrimental and most practitioners favor reducing lateral contacts in restoration design.
Occlusal overload is often referred to in the implant literature as a causative factor in peri‐implant bone loss, or at least a cofactor along with biofilm. Occlusal overload is not listed in the Glossary of Prosthodontic Terms. However, “occlusal disharmony” is listed and occurs when contacts of opposing occlusal surfaces are not in harmony with other tooth contacts and/or the anatomic and physiologic components of the craniomandibular complex. “Occlusal trauma” has also been described as trauma to the periodontium from functional or parafunctional forces causing damage to the attachment apparatus of the periodontium by exceeding its adaptive and reparative capacities. It may be self‐limiting or progressive.79 No evidence currently exists that defines the load‐bearing capability of a dental implant beyond its structural fatigue/load limits.80 Overload was diagnosed in one study based on “the presence of parafunctional habits, radiographs, and clinical complications including screw loosening/fracture or prosthesis factor.”81 Parafunctional habits, such as bruxism, can exacerbate mechanical failure and wear,82 but occlusal load or overload are not necessarily potential causes for late implant failure.
In the completely edentulous implant patient, occlusal overload can occur when the balance between the number of implants, their position, prosthesis design, and parameters of occlusion are not in harmony with the patients’ functional loads, physiological adaptation, and habits. Occlusal overload for these patients is manifested by excessive prosthesis wear, screw loosening/fracture, and occasional implant fracture. Occlusal trauma can exacerbate existing peri‐implant disease to create bone loss (angular or horizontal). Mechanical overload in the completely edentulous implant patient may result in early implant failures when osseointegration has not been established. In cases of late implant failures (after the first 18–24 months), occlusal overload is less likely to be the primary etiological factor.
In the partially edentulous implant patient, there is a wider range of the effect of occlusion depending on the stability and health of the natural dentition, the location of the implant(s) in the arch, the overall occlusal scheme, and whether a mutually protected occlusion exists prior to implant therapy. When dental implants are added to restore overall occlusal stability, the significance of occlusion is elevated. Dental providers must recognize and modify changes to the partially edentulous implant patient even more acutely than to the completely edentulous implant patient.
A debate centers on whether overloading occurs from normal forces on implants of inadequate mechanical strength, or whether the forces are abnormal on mechanically sound implant designs. Narrow‐diameter implants with internal connections have been initially diagnosed as having early implantitis, only to show implant fracture subsequently. Taylor80 has described these mechanical failures as hoop stresses from tangential stress distribution in the implant body which can be multiplied by axial loads. For external connection systems, similar mechanical failure has been reported at a level equal to the base of the abutment screw within the implant body.
Adjunctive splint therapy is recommended in patients with known bruxism parafunction habits by some practitioners, but has been refuted as effective by one paper recently.83
Trauma
Tissue irritation from trauma related to overzealous oral hygiene techniques, such as that seen with floss cuts,78 toothpick use, and excessive brushing, can mimic peri‐implant mucositis symptoms. Confirmation can be made by requesting the patient to demonstrate their hygiene technique. Modification of technique will usually solve the concern in a few days.
A continuous positive airway pressure machine hood can also place pressure on the maxillary anterior area peri‐implant tissues and create a stomatitis from drying soft tissues. This can result in a chronic erythema around the marginal gingiva similar to that in mouth breathers.
Facial trauma involving implant restorations can result in fracturing of the veneering material, bent and/or broken screws, or damage to the implant body itself including a bent or fractured interface.84 Soft tissue injury could also occur which may mimic peri‐implant mucositis, although there may be underlying alveolar fractures which are difficult to visualize clinically or radiographically. Although there are limited publications in this area, it appears that due to the elasticity of the bone encasing the implant and osseointegration, the damage is often limited to the restoration.85
Growth
Incomplete growth and development of younger patients at the time of implant placement can result in future incorrect implant position compared to adjacent natural teeth, especially in the esthetic zone, due to subsequent changes in vertical facial growth affecting the dentition.86 The best indicators of completion of growth to consider when planning implants in younger patients are referenced in the orthodontic literature. Continued skeletal changes affect implant/teeth position throughout life. The adjacent maxillary anterior natural teeth have been documented to extrude vertically and facially along with their proximal bone and soft tissues, resulting in a change in the abutment–restoration interface. This will result in an increased probing depth adjacent to the implant restoration and associated peri‐implant soft tissue changes. There is no preventative solution for this concern, other than to inform patients of this possible long‐term complication and make adjustments as required.
In the case of the completely edentulous implant patient, increase in residual ridge height of the body of the mandible has been documented in numerous cases directly under the cantilever portion of complete arch prostheses.87,88 This change has been attributed to increased biting force permitted by the dental implant rehabilitation on the internal architecture of the trabeculae, followed by secondary changes to the external cortical portion of the bone (Wolff’s Law). The modern explanation for this phenomenon is referred to as skeletal mechanotransduction,89 a process through which forces or other mechanical signals are converted to biochemical signals in cellular signaling. Clinically, this apposition can severely restrict hygiene access, resulting in tissue hyperplasia and mucositis along the cantilever and surrounding the terminal implant. As a result, the design of the intaglio surface of the cantilever should permit long‐term adjustment.
Restoration design
The design of implants and components continues to evolve. From single (one‐piece) implants to multi‐stage implants and back, a multitude of innovations and inventions has occurred. Superiority of connection interfaces and implant surfaces have been hotly contested but lack long‐term evidence.
Intrusion of natural teeth splinted to implants has been mentioned in the literature and connecting natural teeth and implants is generally not recommended.90,91 Use of individual copings and provisionally cemented restoration with nonrigid connectors may be indicated to facilitate retrievability.92 Several authors have demonstrated short‐ to medium‐term stability with teeth splinted to implants.93
Dental implants in partially edentulous patients are “immovable” objects surrounded by an ever changing dentition due to lifelong cranial growth. This phenomenon has resulted in change to the integrity of implant–tooth interproximal contacts, with resultant open contacts, food impaction (trauma), and soft tissue sequelae, such as peri‐mucositis. For this reason, restoration design which allows retrievability and modification of interdental contacts is recommended, particularly for younger patients, those patients with poor occlusal stability, and posterior areas which will exhibit more total arch drift than anterior teeth.
Esthetic discoloration of circum‐implant tissues
Circum‐implant “titanium tattooing”, resulting from mechanical failure of zirconia internal‐connection implants, is a potentially serious complication, especially in the esthetic zone. Long‐term studies of zirconia custom abutments have yet to appear. This complication has been linked only to internal‐connection implants with zirconia abutments.94 Alternative designs with a separate titanium interface have not demonstrated this complication to date.
Drug interactions
Although some authors have listed a significant risk for bisphosphonate‐related osteonecrosis of the jaw (BRONJ), which would be manifested as chronic peri‐implant mucositis, there does not appear to be a statistically significant risk for oral bisphosphonate therapy compared with intravenous bisphosphonate therapy.95,96 A prolonged and close follow‐up period has been recommended for these patients.97
Selective serotonin‐reuptake inhibitors (SSRIs) are widely prescribed drugs in the treatment for depression, which have been reported to increase risk of bone fracture. Failure rates of SSRI patients were double that of nonSSRI patients, although small implant diameters and smoking were associated risk factors. A careful surgical treatment planning for SSRI users was recommended.98,99
Alcohol
The effect of alcohol on dental implant osseointegration has been reported in animal studies and indicates that a decreased bone–implant direct contact will result from excessive alcohol consumption.100,101 Severe alcoholics have a high risk of periodontal disease which would be expected to extend to peri‐implant diseases.102 There are no published articles relating the effect of recreational or illicit drug use on dental implant treatments or peri‐implant diseases. However, the clinician would be prudent to suspect other etiologies for chronic unresolved peri‐implant diseases not listed in the patient’s medical history.
Tobacco
A tobacco smoking habit has been implicated as a significant risk factor in peri‐implant disease since the original Brånemark research was published. When comparing machined‐ (smooth) or rough‐surface implant success, it appears that smoking is a risk factor in smooth‐surface implants only.103 Recent evidence points to an electrochemical change to the surface of the commercially pure titanium implant resulting from exposure to caffeine and nicotine, which leads to greater biofilm accumulation and increase in peri‐implant disease.104 Smokers are at higher risk for peri‐implant mucositis and peri‐implantitis by shaping the peri‐implant microbiomes, even in states of clinical health, and supporting a pathogen‐rich community which may indicate a need for personalized therapeutics.105
Systemic and oral conditions
Hyposalivation and xerostomia can be related to aging, but is often a drug‐mediated condition.106 A patient with three or more chronic diseases requiring drug intake is considered multi‐morbid. Polypharmacy is commonly identified with xerostomia and its associated dental sequelae.
Cognitive impairment is a factor in reduced control of biofilm which can escalate to an increased risk of aspiration pneumonia in patients with dysphagia.107 Although the natural dentition appears to be affected by rampant caries rates in patients with reduced salivary flow,108 dental implants appear to be a successful treatment alternative for the failing dentition.109 Autoimmune disorders, such as Sjogren’s syndrome, can be challenging to manage clinically with the added difficulty of dry oral epithelium and the increased effects of biofilm.110 However, there are scarce publications as to the long‐term prognosis of implants in these patients.
The effect of aging and increased frailty is particularly significant, considering the rate at which the world population is aging. Whether the frail state exists rapidly through a sudden incident or acute disease, or through a slow and chronic pervasive change, the pathway requires intensive health maintenance over a prolonged and unpredictable period. Prosthodontic preparation for patients in the frail state should begin early and should consider access to preventative procedures and simplicity of maintenance.111,112
Diabetes
Diabetes has been proven to be a significant risk factor and etiology of peri‐implant diseases, specifically related to impaired/atypical osseous wound healing.113,114 Clinically, it can be expected that type I diabetics will be less predictable in their overall peri‐implant health management and will require more close follow‐up than type II diabetics. There is short‐term evidence to indicate that well‐managed type 2 diabetes patients manage peri‐implant health equally to nondiabetic patients, if controlled ranges of glycemia, assessed by monitoring HbA1c levels, are maintained.115
Gingival biotype
Mucosal thickness and degree of keratinization in peri‐implant tissues may determine susceptibility to biofilm and debris accumulation and may deter patient compliance with oral hygiene procedures due to chronic irritation.116 Although a number of authors have recommended specific soft tissue dimension and morphology to minimize peri‐implant diseases, there is no long‐term evidence to support a particular philosophy.117,118 There is emerging evidence that a thick gingival biotype, which could be pre‐existing or develop through surgical augmentation as site development or in conjunction with implant placement, is favorable over a thin gingival biotype. Patients need to be informed this is a potential adjunctive requirement for long‐term peri‐implant tissue stability.119,120
Epidemiology
Peri‐implant disease has been reported in numerous publications and is currently an area of interest due to the widespread increased acceptance of dental implants by the profession and the public. There continues to be disagreement in the profession as to the classification and levels of peri‐implant diseases which prevents meta‐analysis of the majority of the publications. For the few papers that survive meta‐analysis, the indicators are that peri‐implant mucositis occurs at a rate of 8–68% and peri‐implantitis occurs at a rate of 1–47%.121–125 It has been reported that patients with a history of periodontitis have almost a sixfold higher incidence in the development of peri‐implantitis than those without periodontal disease.126 The wide variations in epidemiological studies have been attributed to patient variables, clinician experience, implant hardware design, quality of prosthetic reconstruction, and conflicting definitions for the threshold of peri‐implantitis. 127 Nonetheless, epidemiological reports are useful to clinicians and researchers to elevate their awareness of peri‐implant diseases and ensure adequate processes are in place for diagnosis and appropriate management.
Future directions in peri‐implant disease research
As technology continues to evolve, histological assay of various cytokines in peri‐implant sulcular fluid is emerging as a research tool to refine the threshold for mucositis and implantitis.128–130 Higher levels of specific cytokines appear to be linked to the inflammatory process of peri‐implantitis. Inducing synthesis of other cytokines may improve the prognosis of an implant.131 It has been suggested that histological assay should be completed prior to initiating clinical treatment.132 Meta‐analysis of peri‐implant crevicular fluid studies link a definitive diagnosis of peri‐implant infection to inflammatory mediators but do not discriminate between peri‐implant mucositis and peri‐implantitis.133 Animal studies have demonstrated an increase in cytokine production in gingival and bone tissues when stimulated by titanium ions which may promote alveolar bone resorption.134,135 The corrosion of implant materials is considered a dynamic interface through the healing period and is modified by oxidizing radicals created in metabolic processes.136 The effect of long‐term corrosion of titanium and titanium alloy dental implant surfaces in humans has yet to be connected to cytokine production. There remains a significant gap between reported research tools and clinical application.
Treatment
Nonsurgical treatment for PIM or PI
Following a tentative diagnosis, a decision to proceed with either nonsurgical or surgical treatment must be made. Nonsurgical treatment for peri‐implant mucositis is appropriate, generally simpler, more manageable, sufficient for detoxification, and well accepted by the patient. The early detection of peri‐implant diseases is essential as the treatment of peri‐implantitis is not predictable, at times complex, difficult to perform, and nonsurgical therapy has proven to be ineffective.137
The goal of nonsurgical treatment is to control the infection and inflammation by detoxification of the implant surface, and reduction of peri‐implant soft tissue inflammation. The literature shows there are many different, and effective, treatment modalities to consider in detoxification of affected implant surfaces.138 A systematic review and meta‐analysis concluded that long‐term maintenance care for high‐risk groups is essential and informed consent for such patients must include ongoing maintenance therapy.139
The goal of nonsurgical management of biofilm or iatrogenic causes of peri‐implant diseases is to reduce inflammation by appropriate nonsurgical mechanical debridement, using instruments that do not abrade titanium (implant plastic, resin, or carbon fiber curettes and scalers, or ultrasonic scalers with a nonmetallic tip). Adjunctive therapies include use of local or systemic antimicrobial or antibiotic agents following appropriate identification of putative pathogens, possibly with use of bacterial assay. Corrective restorative procedures to reduce sources of food impaction and recontouring of restorations to promote oral hygiene access and encourage peri‐implant tissue health should be included. Debridement and disinfection of the intaglio and cameo surfaces of removable prostheses, and any mechanical attachments, must not be ignored.
The emphasis on patient homecare effectiveness around the peri‐implant tissues is fundamental, irrespective of implant design and manufacturer. Improvement in plaque control is considered to be integral in the maintenance of peri‐implant tissue health and in promoting reduced pocket depth. An in vitro study supported maintenance procedures which leave the implant neck (abutment) surface unaltered or result in reduced roughness are recommended over procedures that increase surface roughness.
Mechanical debridement
Using manual or ultrasonic instruments (with plastic coating) to clean the implant or abutment surface has a potential to affect the implant surface and/or abutment surface, therefore nonmetal scalers or curettes (carbon, plastic, resin‐reinforced, resin‐unreinforced instruments), or titanium instruments, are generally recommended. Metal curettes can alter surfaces and remove superficial material,141 resulting in an even rougher surface.142
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


