22
The Application of Lasers in Orthodontics
Summary
As an adjunctive procedure, soft tissue laser surgery has helped many orthodontists elevate the level of patient care by increasing treatment efficacy, improving oral hygiene around fixed appliances during orthodontic treatment, and enhancing final smile esthetics. Specifically, soft tissue lasers have numerous applications in the orthodontic practice, including: gingivectomy and gingivoplasty, flattening of bulbous papillae, frenectomy, exposure of partially erupted teeth, uncovering temporary anchorage devices, operculectomy, ablation of aphthous ulcerations, and even tooth-whitening. This chapter will review the two most popular lasers used in orthodontics: the diode laser and the solid-state laser, and provide an overview of laser physics, equipment set-up, choosing an appropriate anesthetic, proper laser technique, billing and insurance codes, and laser safety.
Definition and Laser Physics
LASER is an acronym for ‘light amplification by stimulated emission of radiation’. Fundamentally, a laser beam is a focused source of electromagnetic radiation, or light-energy. Laser light energy is defined by three properties:
- Monochromatic (of one color or wavelength)
- Directional
- Coherent.
Simply, a laser beam is a concentrated source of light energy composed of one wavelength, which travels in a specific direction, and all wavelengths of the laser light travel in phase. These properties differ from ordinary light, which is diffuse and non-coherent, allowing laser light energy to target accurately and with high intensity.
The acronym ‘laser’ actually describes the physics behind how a laser beam is created. A laser machine releases light-energy whenever the laser medium is stimulated. Stimulation of the laser medium causes one of its electrons to drop from a higher energy state (Q1) to a lower energy state (Q2), releasing light energy – a process referred to as stimulated emission of radiation. The energy released by the laser medium becomes amplified before exiting through a collimated tube to provide a concentrated source of light energy – the laser beam (Moritz, 2006).
Components of a Laser
All lasers consist of three basic components:
- The laser medium (sometimes referred to as a gain medium)
- The pump source
- The optical cavity or optical resonator.
Laser Medium
The laser medium is the ‘active element’ which produces the laser beam. Most elements in the periodic table can be used as media to develop a laser beam. A laser medium can be a gas, dye (in liquid), solid-state element (distributed in a solid crystal or glass matrix), or semiconductor (diode). The medium will determine the wavelength output, which primarily influences the efficacy of the laser at the target site.
Pump Source
The pump source ‘stimulates’ the lasing medium until light-energy is emitted. Examples of pump sources include: electrical discharges, flash-lamps, arc-lamps, or chemical reactions. The type of pump source used depends on the type of laser medium.
Optical Cavity or Resonator
The laser optical cavity or resonator amplifies the light-energy. The optical cavity is a compartment of mirrors that contains the laser medium. Light-energy released from the laser medium is reflected by the mirrors back on to itself (referred to as feedback), where it may be amplified by stimulated emission before exciting the cavity. The alignment of the mirrors with respect to the laser medium will determine the exact operating wavelength of the laser system.
In summary, a laser is a special form of artificial light with specific properties. A laser beam is produced within a laser machine when the pump source stimulates the laser media, releasing light energy which amplifies as it travels through the optical cavity. The amplified light energy released from the machine is what we refer to as the laser beam. When light energy enters the target tissue, it transforms into heat – a process known as the photothermal effect – resulting in the vaporization of the target tissue cells.
Thermal Ablation
Unlike a scalpel which slices, a laser separates tissue by thermal ablation. Thermal ablation is an instantaneous process of absorption, melting, and then vaporization, resulting in decomposition of the tissue. As the target cells absorb the concentrated light-energy of the laser beam, the tissue rapidly rises in temperature. The target cells instantly undergo stages of warming, welding, coagulation, protein denaturization, drying, and vaporization via a micro-explosion known as spallation (Sarver and Yanosky, 2005).
Thermal ablation is dependent on the amount of light energy absorbed, which is determined primarily by the wavelength of the laser. The degree of tissue absorption is influenced by:
- Laser wavelength (measured in nanometers) – a component of the laser media
- Electrical power of the surgical unit (measured in watts)
- Exposure time
- Composition and thickness of the tissues.
In summary, lasers separate tissue by thermal ablation. The type of laser media produces a specific wavelength, which among other factors, influences the degree of tissue absorption and thus the ‘cutting’ power of the laser.
Historical Perspective
As early as 1917, American physicist Albert Einstein first proposed the theory of ‘stimulated emission’, the process which made lasers possible. In 1954, Charles Townes at Columbia University demonstrated a working device using ammonia gas as the active medium that produced microwave amplification and the first generation of electromagnetic radiation by stimulated emissions. This device was called the ‘maser’ which is an acronym for microwave amplification by stimulated emission of radiation. In 1958, Arthur Schawlow, Townes’s brother-in-law, proposed the operation of optical and infrared masers, or ‘lasers’ – a term first coined by physicist Gordon Gould in 1957.
In 1960, the first laser to use visible light (using a ruby medium) was developed by physicist Theodor H Maiman, following the theoretical work of Einstein, Townes, and Schawlow. In 1962, Robert Hall developed the first diode or semiconductor laser. The carbon dioxide gas laser was invented by Kumar Patel in 1964, and 4 years later this laser was used to perform the first soft tissue surgery.
In 1985, Paghidiwala, tested the erbium-doped solid state laser (Er:YAG) on dental hard tissue. Nearly a decade later, in 1997, the United States Food and Drug Administration (FDA) approved the Er:YAG solid-state laser for hard tissue surgery. The following year (1998), the first diode laser was approved for soft tissue surgery. More than 25 years since Dr Paghidiwala first tested a laser on dental tissues, dental laser technology has undergone remarkable advancements in design. Contemporary dental laser machinery has become portable, compact, wireless, safer, increasingly affordable, and simpler to operate, allowing more orthodontists to incorporate soft tissue laser surgery into their practice.
Laser Versus Scalpel
A laser offers numerous advantages compared with conventional scalpel surgery. The most significant advantage is that a laser coagulates capillaries, seals lymphatics, and sterilizes the surgical field during ablation (Sarver and Yanosky, 2005). Separation of tissue is more precise with a laser than a scalpel (Rossman and Cobb, 1995). Additionally, minor aphthous and herpetic ulcerations – which commonly occur during the early stages of treatment as the patients get accustomed to their orthodontic appliances – can be vaporized. Laser surgery is routinely performed using only light local anesthetic or compound topical anesthetic. Furthermore, there is markedly less bleeding with laser surgery, particularly for frenal surgery, as well as minimal edema, and no need for sutures or unsightly periodontal dressing (Haytac and Ozcelik, 2006). A report suggested that laser excisions produce less scar tissue than conventional scalpel surgery (Fisher et al., 1983), although contrary evidence also exists (Buell and Schuller, 1983; Frame, 1985). Post-surgically, patients report less discomfort, fewer complications related to speaking and chewing, and require less pain medication than do patients treated with conventional scalpel surgery (Haytac and Ozcelik, 2006). The benefits of laser surgery are best summarized by Sarver and Yanosky (2005): ‘[soft tissue lasers] result in shorter operative time and faster post-operative recuperation.’
The primary disadvantage of laser surgery is the operatory and upkeep expense. Some clinicians have reported additional disadvantages, such as: less tactile sensation, tissue desiccation, and poor wound healing (Baker et al., 2002). Furthermore, laser surgery is primarily excisional and performed without a flap, often resulting in no change in alveolar crest height. As such, there is a tendency for significant tissue rebounding or regrowth after laser surgery.
Diode Versus Solid-State Lasers
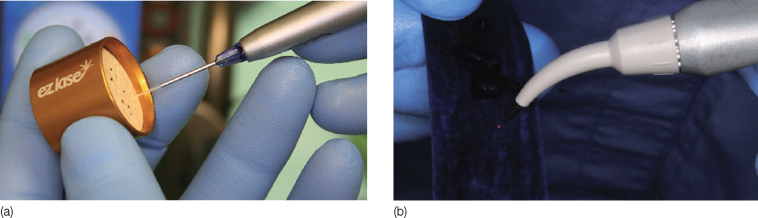
The two most popular lasers used in dentistry are the diode and the solid-state lasers (Figure 22.1). Diode lasers are almost exclusively used for soft tissue surgery. Solid-state lasers, on the other hand, can be used for both soft and hard tissue surgery, such as tooth preparation, root canal debridement, and crown lengthening. The fundamental difference between a diode and a solid-state laser is the laser medium, which generates laser beams of different wavelengths. As already stated, the laser medium determines the wavelength output, which ultimately influences the efficacy of thermal ablation at the target site.
Figure 22.1 (a) Ezlase 940 diode laser and (b) Waterlase MD Turbo (Er,Cr:YSGG) erbium-doped solid-state laser (Biolase).
Images are not to scale.

Diode (Semiconductor) Lasers
Diode lasers convert electrical energy into light energy. Diode lasers are known as semiconductors, as they use a media of gallium and arsenide, and occasionally indium and aluminum, whose ability to conduct electricity is between that of conductors and insulators. By doping the laser medium with impurities (dopants), stimulated emission occurs.
The wavelengths produced by diode lasers range between 810 nm and 980 nm. Light energy at these wavelengths is easily absorbed by melanin (soft tissue pigmentation) and hemoglobin, and poorly absorbed by enamel. Therefore, diode lasers are highly effective in soft tissue ablation, hemostasis, and sealing lymphatics, with low risk of damaging teeth and bone, making them ideal for soft tissue laser surgery (Kravitz and Kusnoto, 2008). Compared with other laser types, diode lasers are compact, reliable, and have a long operatory lifetime, and are packaged in portable units typically weighing less than 4.5 kg (10 lb). Connecting to the main unit is a thin, pencil-sized hand-piece containing a 200–400 µm fiberoptic tip. Newer models have handpieces that receive single-use, twist-on laser fiberoptic tips, providing a higher potential standard of cleanliness and eliminating time-consuming stripping and cleaving of the fiberoptic tip.
Priming
Before surgery with a diode laser, the fiberoptic tip must be conditioned or primed. All diode lasers need to have some type of pigment applied to the fiber tip in order to create a sufficient amount of energy for ablation. Priming is the process of concentrating heat energy at the fiberoptic tip (Tracey, 2005). Priming is performed by tapping an initiated-fiberoptic tip on thick blue articulating paper (Figure 22.2), a felt tip marker, a solid color in a magazine page, or a cork. Essentially, the laser tries to ablate the pigmented region, creating a super focus of light energy. Failure to properly prime the diode laser may result in less effective tissue ablation.
Figure 22.2 Priming a diode laser with a cork or thick, blue articulating paper. (a) EZlase 940 and (b) Odyssey Navigator (Ivoclar Vivadent).
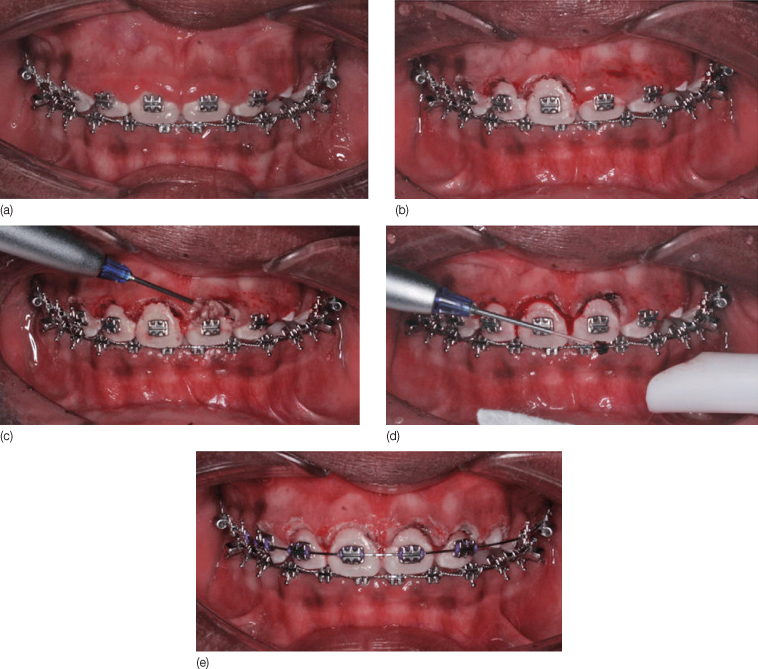
During laser surgery with a diode, the fiberoptic tip should be held in light-contact with the tissue. For the majority of surgeries, soft tissue ablation is performed at 1.0–1.5 W, with gentle, sweeping brush strokes. All lasers are collimated; as such, the ‘cutting’ end is at the tip. Therefore, dragging the laser sideways tends to collect soft tissue build-up and may even damage the fiberoptic tip. During surgery with a diode laser, tissue margins may appear dark and charred. High-speed suction is critical to remove laser plume and burnt tissue smell, as well as to maintain a clear field of vision (Figure 22.3).
Figure 22.3 (a–e) Removal of excess tissue due to poor oral hygiene. (d) Dragging the fiberoptic tip sideways can lead to tissue build-up which will need to be removed with a 2 × 2 gauze. High-speed suction is critical to eliminate laser plume.
Solid-State Lasers
Solid-state lasers use a gain medium that is a solid, rather than a liquid or gas. It should be noted that semiconductor lasers are also in the solid state, but are considered in a separate class from solid-state lasers. The active laser medium in a solid-state laser consists of a glass or crystalline matrix. Two common matrices are the yttrium aluminum garnet (YAG) and the yttrium scandium gadolinium garnet (YSGG). Comparative studies have shown little difference in efficacy between the two (Harashima et al., 2005). Atoms in the crystal are excited to produce light energy when dopants, such as erbium, chromium, and neodymium, are added to the medium (i.e. Nd:YAG, Er:YAG, or ErCr:YSGG). Erbium-doped solid state lasers are most commonly used in dentistry (Kravitz and Kusnoto, 2008).
The wavelengths produced by erbium-doped solid-state lasers range between 2780 nm and 2940 nm. Unlike diode lasers, light energy at this wavelength is easily absorbed by hydroxyapatite and surface tissue water, and therefore can ablate both hard and soft tissues. Erbium-doped solid-state lasers are routinely used by pediatric dentists and general dentists for caries excavation, and less frequently for endodontic and periodontal procedures. When performing soft tissue laser surgery with an erbium-doped solid-state laser, the laser machine is operated at low electrical power to reduce the depth of tissue penetration (Kravitz and Kusnoto, 2008).
For most gingival surgeries, soft tissue excision may require 1.5–2.5 W depending on the tissue thickness. Coagulation with a solid-state laser requires a different setting, generally less than 1.0 W often without water spray. It should be noted that a solid-state laser will begin to ablate hard tissue at approximately 4.0–5.0 W. Solid-state lasers are packaged in larger, more complex rolling units (weighing up to 40 kg or 90 lb). The laser handpiece resembles a high-speed handpiece with removable fiberoptic tips ranging from 400 µm to 750 µm.
During surgery with an erbium-doped solid-state laser, the fiberoptic tip should be held 1 mm away from the tissue (Hadley et al., 2000). Priming is not required as the solid-state lasing medium does not absorb pigmentation. Excision is performed with slow, short back-and-forth strokes. Coagulation is achieved under a different operatory setting, with low wattage and often no water. Tissues appear slightly reddish during excision and chalky white after coagulation. Although a solid-state later can effectively control hemorrhaging, hemostasis may be easier with a diode, particularly during more invasive soft tissue surgeries such as frenectomies.
Choosing a Proper Anesthetic
Soft tissue lasers both coagulate and produce a mild anesthetic effect during ablation. Accordingly, many clinicians perform soft tissue laser surgery using only light local anesthesia, strong topical anesthesia, or without any anesthesia at all. Local infiltration with 2% lidocaine, 4% articaine, or occasionally 3% mepivacaine (in patients with contributory heart conditions) can be limited to the specific surgical site, i.e. interpapillary, interligamentary (the periodontal pocket), interseptal or directly into the frenum or operculum. The injection can be performed using a short or long 27 or 30 gauge needle. This method is often relatively painless, requires very little anesthesia, and is useful for anxious children who may not tolerate conventional local anesthetic technique. However, some clinicians desire a more profound anesthetic effect and prefer superior alveolar nerve blocks at the height of the mucobuccal fold.
Debate Regarding Compound Topical Anesthetics
There has been a growing interest among orthodontists and pediatric dentists in the use of strong topical compound anesthetics to be used in place of local infiltration. Compounding is the process by which the pharmacist or dentist combines, mixes, or alters pharmaceutical ingredients to create an individualized medication in accordance with a prescription (United States Pharmacopeial Convention, 2004). Essentially, compound topical anesthetics are nonregulated, custom-made, strong topical formulations.
Compound topical anesthetics are often highly viscous to prevent against run-off, include several active anesthetic agents to provide a wide spectrum of anesthetic action, and contain a vasoconstrictive agent. Specifically, common formulations include high concentrations of both amide (lidocaine and prilocaine) and ester (tetracaine) anesthetics, and small dosages of the nasal decongestant phenylephrine. Popular compound topical anesthetics, such as TAC 20% Alternate (20% lidocaine, 2% phenylephrine, 4% tetracaine) and Profound PET (10% lidocaine, 10% prilocaine, 4% tetracaine, 2% phenylephrine) are widely used by orthodontists for soft tissue laser surgery and placement of orthodontic temporary anchorage devices. These topical local anesthetics are contraindicated in elderly patients, those with hypersensitivity to ester- and amide-type local anesthetics, para-aminobenzoic acid (PABA) allergies, severe hypertension, hyperthyroidism, or heart disease. To date, compound topical anesthetics such as TAC 20% Alternate and Profound PET are neither FDA regulated nor unregulated drug products (Kravitz, 2007).
Though highly effective, concern exists regarding the safety of these anesthetics (Jeffcoat, 2004). The risks regarding use of compound topical anesthetics are the following:
- Not-regulated by the Federal Food Drug and Cosmetic Act
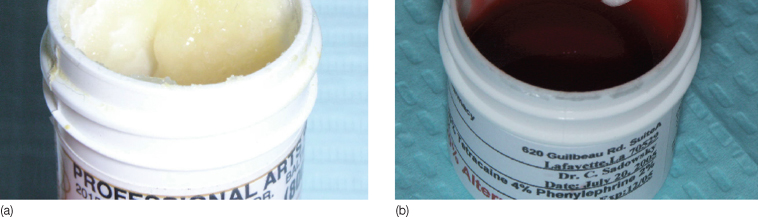
- Vials may be improperly mixed, measured, or labeled (Figure 22.4)
- Maximum recommended dosage (MRD) is unknown as they are intended for individual use only
- Low therapeutic index – a narrow difference between optimal dose and toxic dose
- Often contain high concentrations of ester anesthetics, which may lead to PABA anaphylaxis.
Figure 22.4 (a,b) Variability of compound topical anesthetics. Two photos of the same common-name anesthetic TAC 20% Alternate ordered from different compounding pharmacies. Note the significant difference in quality of mixture. Since compound topical anesthetics are not federally regulated, vials may be improperly labeled.
Doctors who store and repeatedly use the same compound formulation on multiple patients may be in violation of state and federal laws. To date, two deaths have been attributed to the lay use of excessive amounts of compound topical anesthetics. Clinicians who are intent on using compound topical anesthetics may consider the following protocol.
1. Review medical history to ensure no contributory health conditions.
2. Dry the mucosa with 2 × 2 gauze.
3. Apply 0.2 mL (equivalent to one cotton swab head) of topical anesthetic to the mucosa.
4. Let the topical anesthetic remain on tissue for 3–5 minutes while the doctor remains with the patient. Prolonged application can cause tissue-irritation and sloughing.
5. Suction away topical and confirm anesthesia with a periodontal probe. Anesthetic effect will last approximately 30 minutes.
Despite the risks, there is arguably a place for doctor-prescribed, doctor-applied compound topical anesthetics for use sparingly on an individualized basis. However, until these drugs become federally regulated, the large-scale pre-production of popular formulations by big pharmacies remains an end-run on manufacturing requirements, and their routine multi-patient use by either orthodontists or other dental specialists remains a questionable therapeutic practice.
Laser Machine Set-Up
All soft tissue lasers have similar machine components and set-up steps. The main components of a dental laser machine include:
- A touch-screen control system, which incorporates factory loaded pre-sets and customizable settings such as watts, joules, and pulse repetition rates
- A handpiece with a fiberoptic tip
- Foot pedal or footswitch to initiate laser firing
- Protective goggles for the patient, the doctor, and the assistant, which blocks a range of wavelengths specific to the laser.
First, connect the power cable and footswitch, and then place a sterilized fiberoptic tip on the handpiece. Second, turn on the machine and use the touch-screen window to control the appropriate settings. Ablation of thicker tissue may require increased wattage, whereas surgeries with a likelihood of bleeding such as frenal surgery or inflamed tissue may respond better to continuous-mode laser firing rather than pulsed-mode. Erbium-doped solid-state lasers typically connect to the air-pressure valves in the chairside delivery unit and may require distilled water. Third, prepare to initiate laser firing by switching the laser from ‘standby’ to ‘ready’ mode. Finally, initiate laser firing by pressing on the footswitch. If needed, prime the laser tip at this point prior to entering the mouth. Remove foot from the pedal to cease laser initiation and move the handpiece to the mouth />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


