Tooth organogenesis, morphology and physiology
Tooth development
The “intelligence” or “activating force” that directs the precise coordination of multiple cell line activity, growth, migration, induction, fusion and disintegration with such control and symphonic grace, still eludes us. In our current state of knowledge, we are left merely to describe the observable and timed changes gleaned through various biological studies. Experimental studies also give us some insight about the genomic and proteomic involvement in the process, even though the picture is far from complete. Yet there is already sufficient intuitive knowledge to enable the culture of tooth tissues and whole teeth in the laboratory, albeit in a neophytic way (Fig. 1.1).
Early development of teeth
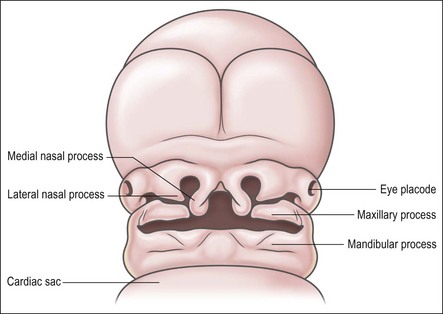
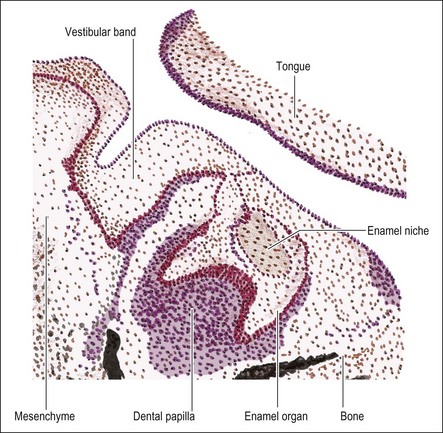
The primitive mouth cavity is evident as a slit-like space lined by ectoderm in the 3–4-week-old human embryo. It is located under the surface of the brain capsule and above the pericardial sac where the heart forms. The mouth cavity is still separated from the primitive pharynx by the oropharyngeal membrane. The mandibular processes grow ventrally on each side of the head to meet gradually in the midline, where they form the lower border of the mouth opening. The maxillary processes arise from the upper surfaces of the origin of the mandibular process and likewise grow towards the midline, to form the upper border of the mouth below the brain capsule (Fig. 1.2). The maxillary and mandibular processes are essentially extensions of mesenchyme tissue covered by ectoderm. The ectoderm is a layer of low columnar epithelial cells, resting on a basal lamina which separates them from the mesenchymal tissue, which originates from the neural crest cell line. In some regions, such as the tooth-bearing part, the epithelium has a more superficial part, which consists of 2–3 layers of flattened cells. At this stage, the maxillary and mandibular processes do not show separate lip or gum regions; the development of the lips, cheeks and gum regions is closely associated with the development of the dental lamina, from which teeth arise.
Primary epithelial band, vestibular band and dental lamina
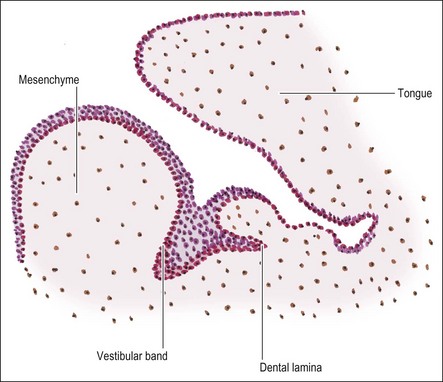
During the seventh week of embryonic life, the primary epithelial band divides on its deep surface into two processes; the outer, thicker one becomes the vestibular lamina (responsible for the later separation of lips/cheeks from gums) and the inner, smaller one becomes the dental lamina (which later gives rise to the teeth) (Fig. 1.3). As the dental lamina grows in length, it penetrates deeper into the mesenchyme; at the front of the mouth in a lingual direction, to form a shelf-like projection and at the back of the mouth remaining more vertical (Fig. 1.4). It is not known whether this results from active invagination of the lamina or upward proliferation of the mesenchyme.
Enamel organs
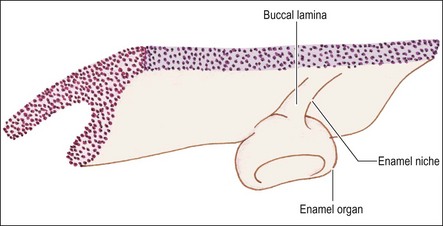
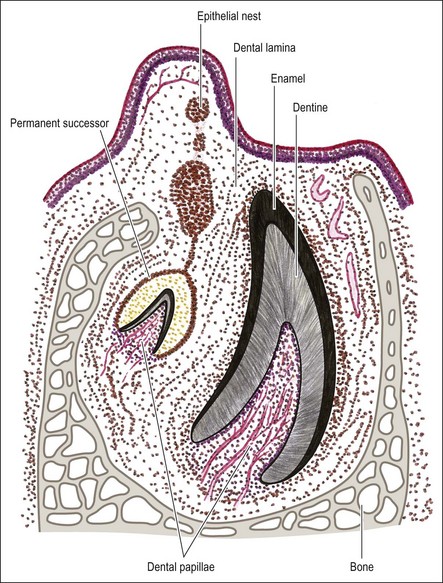
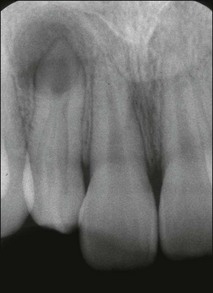
A short while after formation, the dental lamina thickens into small rounded swellings, involving the whole thickness from free edge to the base of attachment to the oral epithelium. These are the enamel organs of the deciduous teeth with four in each quadrant (2 incisors, canine and first deciduous molar) (see Fig. 1.4). The dental lamina continues to grow backwards, giving rise to further enamel organs for the second deciduous molar (10-week embryo), and the permanent molars (first permanent molar at 16-week embryo; second and third permanent molars after birth). At 10 weeks of embryonic life, the enamel organs and dental lamina conform to a catenary curve. As the tooth germs grow, the spacing between them is reduced. There is at this early stage no indication of the successional permanent teeth, which develop later by budding off from the lingual aspects of each deciduous enamel organ.
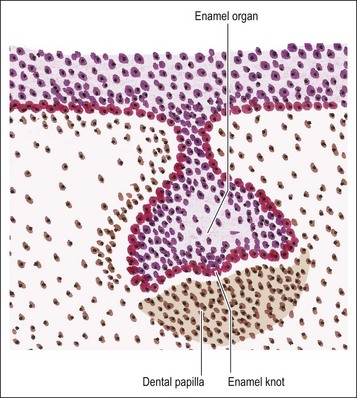
Dental papilla
The mesenchymal tissue surrounding the developing enamel organ responds by proliferation to form a dense mass of cellular tissue. This gives rise to the dental papilla (primitive pulp) and the follicular sac for each tooth bud. The enamel organ in the “bud” stage appears as a simple, spherical to ovoid, epithelial condensation that is poorly morpho- and histo-differentiated. The epithelial component is separated from the adjacent mesenchyme by a basement membrane. The combination of enamel organ, dental papilla and follicular sac are collectively known as the tooth germ (Fig. 1.5). The enamel organ becomes concave on its papillary surface and begins to grow at the rims so as to encircle the dental papilla, which, at this stage, is partly capped by the enamel organ (hence “cap” stage) (Fig. 1.6) and progressively embraces a greater volume of it, to be called the “bell” stage (Fig. 1.7). At the cap stage, the centre of the concavity develops a projection of epithelium called the enamel knot (Fig. 1.6), which soon disappears by programmed cell death (apoptosis) and seems to contribute cells to the enamel cord. The enamel knot represents an important regulatory signalling centre during tooth development by producing bone morphogenetic proteins (BMP-2, BMP-7), fibroblast growth factor (FGF–p21 cyclin-dependent kinase inhibitor), sonic hedgehog (Shh), WNT and transcription factors. These signals regulate growth and development of the epithelial folds that correspond to the cusp pattern of the mature tooth. The primary enamel knot also determines the position of the secondary enamel knots corresponding to the site of the future cusps. The enamel cord is a strand of cells seen at the early bell stage of development. When present, it overlies the incisal margin of a tooth or the apex of the first cusp to develop. It has been suggested that the enamel cord may be involved in the process, by which the cap stage is transformed into the bell stage or that it is a focus for the origin of stellate reticulum cells.
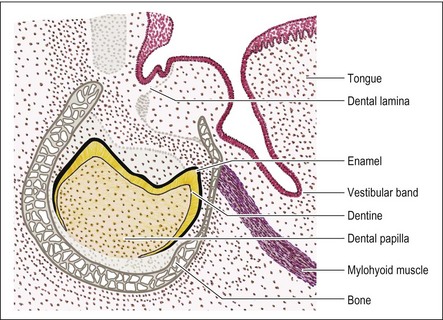
Vestibule formation
Concurrent with the enamel organ development, the vestibular band growth continues apace. At around the time of the cap stage, a vertical cleft becomes established in the vestibular band, separating the formative lips and cheeks from the formative gums (Fig. 1.8). As for the dental lamina, the vestibular band development progresses backwards.
Changes to and further development of dental lamina for permanent molars
As the enamel organ is reaching the cap stage, so the dental lamina lengthens and divides into buccal and lingual parts, though the function of this is unknown. By the time the enamel and dentine formation begins during early bell stage, the dental lamina connecting the tooth germs to the oral epithelium starts to degenerate leaving a network of strands and clumps of epithelial cells. At the same time, the dental lamina continues to grow backwards to give rise to the permanent molars but, by this stage, is separated from the oral epithelium.
Development of successional permanent teeth
The enamel organs for the successional teeth arise so differently from the permanent molars that it raises the question of whether the permanent molars should actually be regarded as part of the deciduous series. During the fourth month of fetal development, an epithelial process appears on the lingual aspect of each enamel organ; this becomes the lamina for the successional teeth (Fig. 1.9), giving rise to tooth germs in like manner to the deciduous teeth.
Differentiation of enamel organs
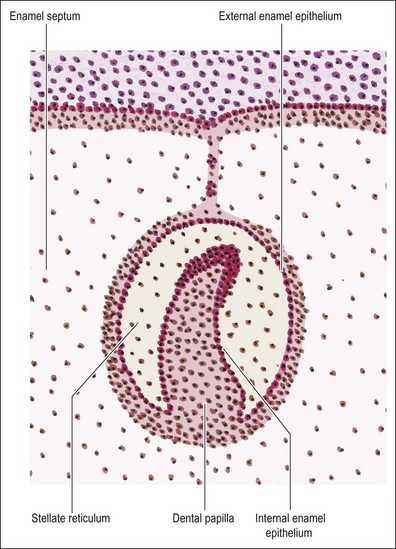
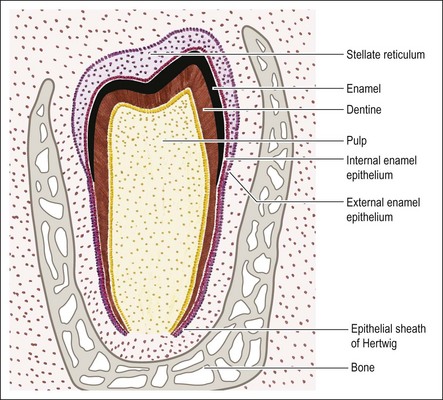
Although the cells of the enamel organ originate from the same source, those at the outer layer appear different in that they are low columnar and continuous with the basal layer of the oral epithelium. The deeper cells are rounder and more closely packed. As the enamel organ enters the cap stage, the outer cells become taller. From this stage, the cells on the outer aspect become differentiated into the “external enamel epithelium” on the outer convex aspect, and the “inner enamel epithelium” on the inner concave aspect. The two layers meet at the rim of the enamel organ known as the cervical loop, which remains an active site of cellular proliferation until the entire tooth is mapped out (Fig. 1.10). It is the epithelial part of the adult stem cell niche.
During the cap stage, the cells within the enamel organ become separated from one another, maintaining contact only at the desmosomal attachments, giving rise to a star-like appearance which gives their new name of stellate reticulum. This delicate and loosely formed tissue gains in volume during the bell stage; the spaces between the cells are occupied by extracellular material with significant quantities of glycosaminoglycans. The function of this tissue appears to be nutritive, particularly before calcification begins and also supportive (physically) during calcification. Also at the bell stage, a further distinct layer of cells becomes evident between the internal enamel epithelium and the stellate reticulum. It is called the stratum intermedium and is 2–3 cells thick. The formation of dentine and enamel begins very soon after this stage is reached; the enamel originating from the internal enamel epithelium, which gives rise to the ameloblasts. As the mineralizing matrices of enamel and dentine are laid down, the shape of the crown becomes fixed.
Differentiation of dental papilla, dental follicle and sheath of Hertwig
Once the enamel organ has mapped out the entire form of the clinical crown, further growth at the cervical loop is geared towards development of the root. From this point onwards, the internal and external enamel epithelia are not separated by the stratum intermedium and stellate reticulum but grow as a two-layered epithelial wall, which is now called the sheath of Hertwig. The sheath of Hertwig maps out the shape of the root(s) (see Fig. 1.10). It is disposed in a simple tube for a single root, and in a more complex form for multiple roots. This is achieved by the inward growth of horizontally directed processes of epithelium.
Differentiation and function of the tooth germ
The fully differentiated enamel organ is depicted in Figure 1.10, and consists of: (1) the external enamel epithelium; (2) the stellate reticulum; (3) the stratum intermedium; (4) the internal enamel epithelium. At the growing rim of the enamel organ, the internal and external epithelia are continuous but separated from the mesenchyme by the basal lamina. This also separates the tooth germ from the vascular tissue of the follicle.
The calcified tissues of the teeth, enamel, dentine and cementum all develop between the internal enamel epithelium (including its root-ward continuation in Hertwig’s sheath) and the dental papilla. The basal lamina separating the internal enamel epithelium and dental papilla represents the enamel–dentine junction, separating the ectodermal derivative, enamel, from the mesenchymal derivative, dentine. Following the epithelial–mesenchymal interactions at the future enamel–dentine junction, the amelogenesis and dentinogenesis occur almost simultaneously. The odontoblasts of the developing dental pulp initiate dentine matrix formation prior to the beginning of amelogenesis. They produce collagen that is formed into bundles pointing towards the cells of the internal enamel epithelium which are, at this stage, known as pre-ameloblasts. Enamel is laid down between the epithelial surface of the basal lamina and the outwardly retreating ameloblasts; the thickness of the enamel being determined by the extent of migration of the ameloblasts. Dentine is laid down between the mesenchymal surface of the basal lamina and the inwardly migrating dentine-forming cells, the odontoblasts, which originate from the dental papilla. The thickness of the dentine is determined by the distance migrated by the odontoblasts. The continual formation of dentine leads to progressive reduction in the size of the pulp cavities and root canals of the teeth. Cementum is laid down on the surface of the dentine that is not covered by enamel after the Hertwig’s sheath starts to break up.
Molecular regulation of tooth development
Tooth development is a very complex process involving many growth factors and transcription factors that help ensure an ordered and controlled development of individual tooth germs, as well as the entire dentition. Epithelial–mesenchymal interactions require signalling between the two major components of the tooth germ. Bioactive molecular signals (i.e. transcription factors, growth factors, cytokines) are produced in a specific spatial and temporal sequence, such that the cascade of events results in a tooth with the appropriate tissues and shape. A model of the molecular regulation of tooth development from initiation to crown morphogenesis (Thesleff, 2003) is given in Figure 1.11.
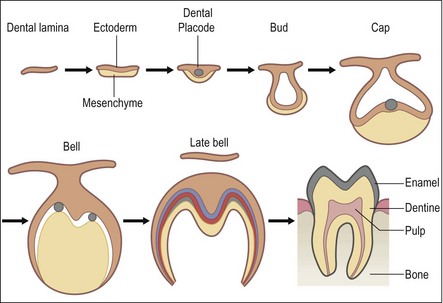
Fig. 1.11 Schematic model of the molecular regulation of tooth development. Signal molecules (BMP: bone morphogenic proteins; FGF: fibroblast growth factors; Shh: sonic hedgehog; TNF: tumour necrosis factor) mediate the interaction between epithelial (green) and mesenchymal tissues (blue) and regulate the expression of genes in the responding tissues (shown in boxes). Signalling centres (red) appear in the epithelium reiteratively and secrete locally many different signals that regulate morphogenesis and tooth shape. (Adapted with permission from Thesleff I (2003) Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci 116(9),1647-8)
Anatomical anomalies
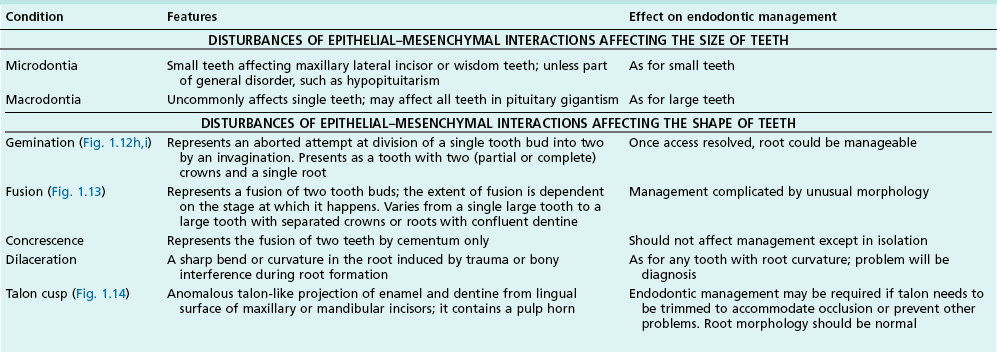
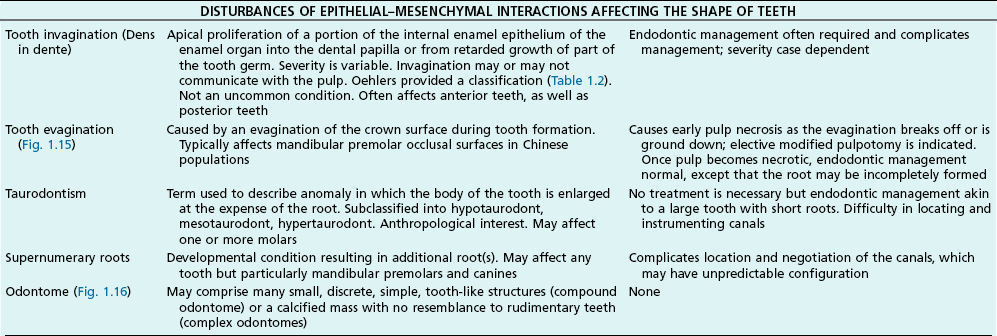
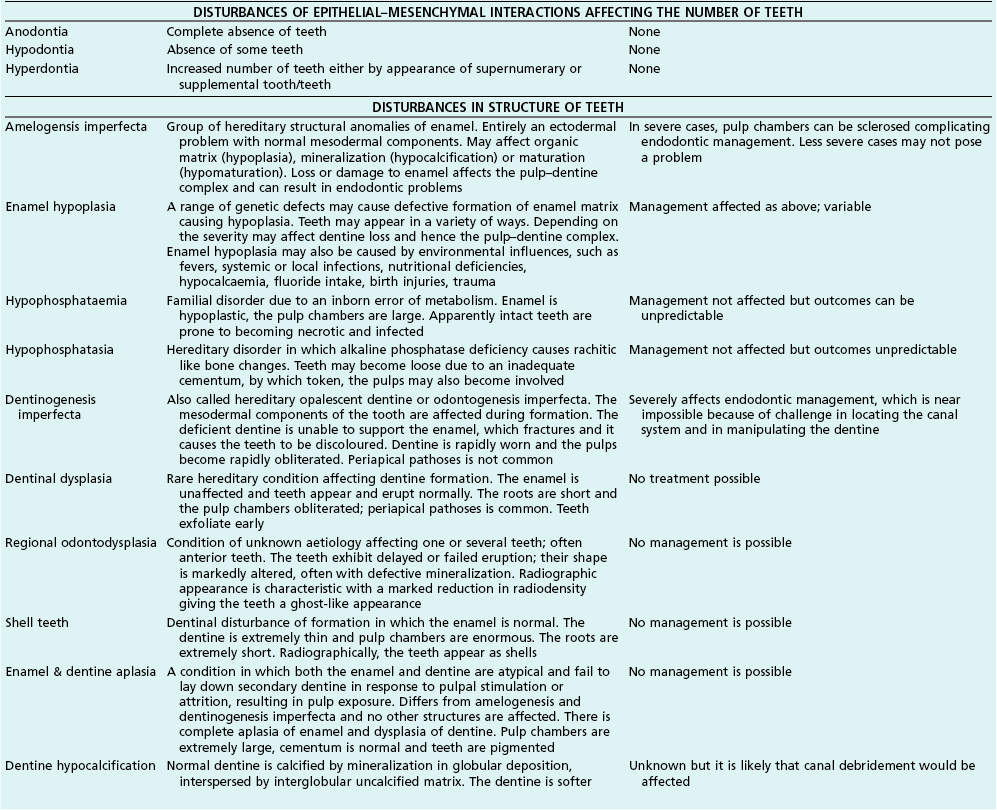
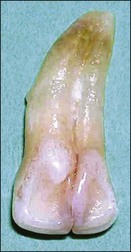
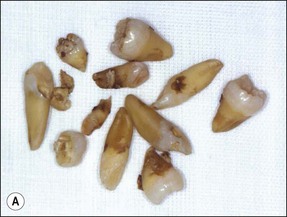
Tooth characteristics such as size, shape, number and structure are genetically determined as evident from the above section. The most stable teeth in the human dentition, showing minimum genetic variation are the canines, central incisors and first molars. The most variable teeth are the maxillary lateral incisors, the second premolars and the second and third molars. Malformed teeth may display bizarre pulp-space configurations. Most commonly this occurs in invagination, evagination, talon cusps, dilacerations and gemination (Fig. 1.12). Table 1.1 below gives a classification of the malformations and a brief summary of the presenting features, which may or may not affect endodontic management. Given the variation in degree of severity, each case must be judged on its own merits as far as management is concerned.
Table 1.2
| Type l | The enamel-lined invagination is of the minor form and confined within the crown; it does not extend beyond the level of the external amelo–cemental junction |
| Type 2 (Fig. 1.17) | The enamel-lined invagination invades the root but remains confined within it as a blind sac. It may, however, communicate with the pulp. The invagination does not breach the periodontal ligament and may or may not be grossly dilated; in the former case, there is often a corresponding dilation of the root or crown |
| Type 3a (Fig. 1.18) | The lined invagination penetrates through the root and opens apically or laterally at a foramen, sometimes referred to as a “second or pseudo foramen”, in the root. There is usually no communication with the pulp, which lies compressed within the wall around the invagination process. The invagination may appear to be completely lined by enamel but, more often, a portion of it is lined by cementum. As in Type 2, there may or may not be a dilatation of the tooth |
| Type 3b (Fig. 1.19) | As in Type 3a but it communicates with the periodontal ligament at the apical foramen without communicating with the pulp |
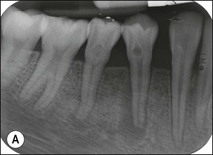
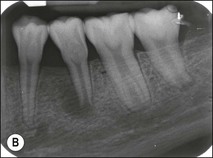
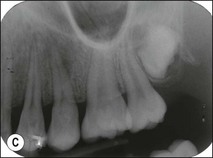
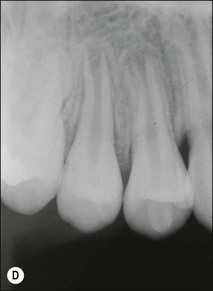
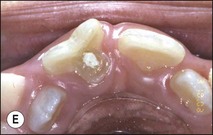
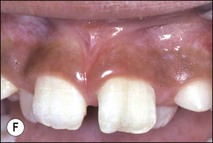
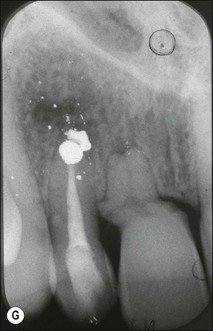
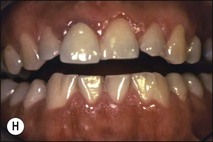
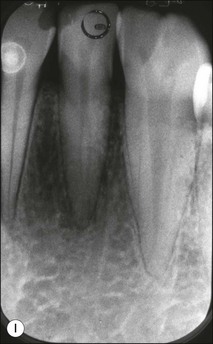
Fig. 1.12 Pulp-space configurations in teeth with developmental anomalies: (a–c) Tooth invagination, (d) tooth evagination, (e,f) Talon cusp, (g) dilaceration (h,i) gemination
Tooth and root shapes
Variation by tooth type
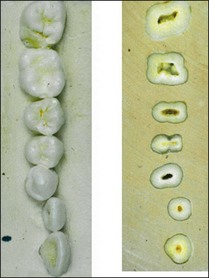
Every dentist is familiar with the characteristics of tooth shape and should be able to identify each tooth type if confronted with extracted teeth, by type and quadrant. Anterior teeth are genetically evolved to help incise food; they have incisal edges and single roots to guide excursive mandibular movements. Posterior teeth are evolved to crush and grind food; they have cusps and multiple roots to support biting loads. The incisive edges of the anterior teeth are in line with the buccal cusps of the posterior teeth. The lingual cusps of the posterior teeth decrease in size towards the front and are represented in the anterior teeth by the cingulum (Fig. 1.20). The canines form the junction or buttress between the anterior and posterior teeth, often taking the majority of the lateral guiding forces, reflected in their longer and bulkier roots.
Variation by race
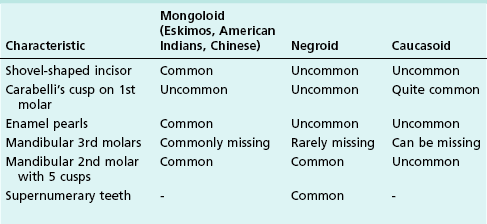
The development of the dental tissues is genetically driven. The growth and migration of the internal and external enamel epithelium in defining the crown and root forms of teeth is particularly heritable. Familial and racial traits affect the three-dimensional spatial configuration of the tooth and pulp space. As roots continue development after eruption and when under functional load, root form may be influenced by functional factors. Most human populations are of very mixed origins, although some communities have remained isolated. Genetic and functional influences have led to the development of characteristic traits in certain populations (Table 1.3). The Australian aborigines and the Eskimos have the largest teeth, while the Bushmen of South Africa and the Lapps have the smallest teeth. The Lapps have long and well-developed roots. In Bantu races of Africa, the mandibular molars increase in size posteriorly. Characteristics such as size, shape and fissure patterns are also genetically determined.
Caucasoid people’s maxillary first and second molars have similar root and canal morphology. The majority have two buccal and one palatal root emerging from a common short root trunk. Anomalous variations, such as fused roots are seen occasionally in first molars but more frequently in second molars. Another less frequently reported variation limited to second molars is the double palatal root/canal. In the three-rooted molars, the majority of palatal and distobuccal roots possess the Vertucci type I canal system (simple, single canal). The complexity of the mesiobuccal roots of first and second maxillary molars was noted by Hess & Zurcher (1925) but only became the focus of more detailed and repeated investigations after the study of Weine et al. (1969). The canal anatomy of maxillary molars of other racial groups is less frequently investigated.
Variation by time
An understanding of the processes that lead to a fully formed tooth helps in differentiating the various patterns or configurations that occur in the anatomy of roots and pulps. Calcification times, eruption and apical root closure dates provide important information in this regard. If eruption dates can be remembered (Table 1.4), as a general rule, the calcification of the tooth crown is complete 3 years before eruption and root end closure 3 years after eruption.
Table 1.4
| Year | Tooth | |
| 6–7 | 16/26/36/46 | 31/41 |
| 7–8 | 11/21 | 32/42 |
| 8–9 | 12/22 | |
| 9–10 | 13/23 | 33/43 |
| 10–11 | 14/24 | 34/44 |
| 11–12 | 15/25 | 35/45 |
| 12–13 | 17/27 | 37/47 |
| 17–22 | 18/28 | 38/48 |
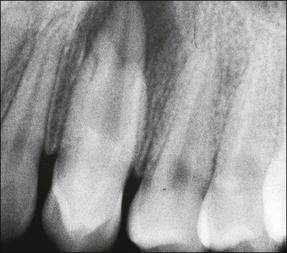
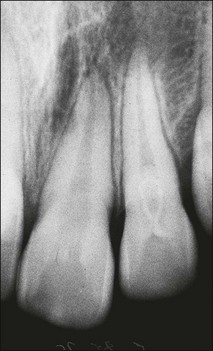
The external form and size of the tooth is clearly fixed at formation but the internal surface continues to change throughout life, even after root maturity, as secondary dentine is laid down continuously and maybe supplemented by tertiary dentine in response to caries, tooth surface loss and occlusal wear. With the passage of time, the pulp volume therefore decreases (Fig. 1.21). This process can occur at different rates in different teeth.
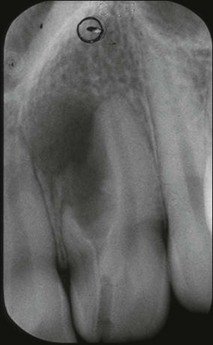
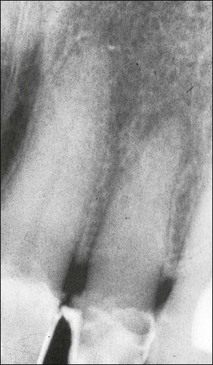
In premolar and molar teeth, laying down of dentine at the roof of the pulp chamber is less than that at the floor of the pulp chamber. In addition to this decrease in the height of the pulp chamber, there is also a decrease in the mesiodistal width of the pulp chamber; however, canals do not become completely occluded or sclerosed. Even though the coronal portion of the canal system may become obliterated, the apical portion usually remains patent. Such canals can be difficult to locate and treat when the remaining pulp becomes infected (Fig. 1.22).
Pulp space and its morphologic patterns
Classifications of pulp systems
In describing the internal anatomy of teeth, it is probably more appropriate to refer to the morphology of the pulp space, which is alternatively known as the root canal system. The pulp space is that part of the pulp–dentine complex which, in a healthy tooth, is occupied by the pulpal tissue, that is, the space within the hard tissue of teeth in which, in health, the pulp resides. Due to the structure of the pulp–dentine complex, this also includes over 20% of the dentine in the form of tubules. In disease, the space becomes the province of microorganisms.
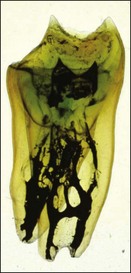
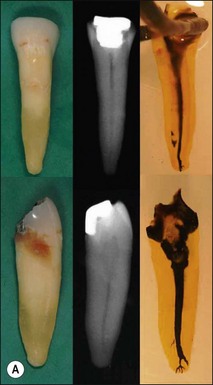
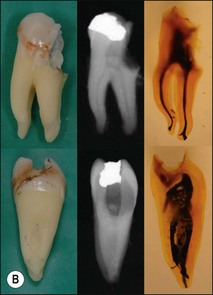
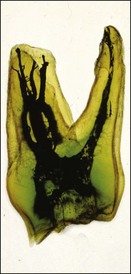
The pulp space is complex and bears little resemblance to the stylized diagrams that are often used to explain the traditional terms used in describing the anatomy of the pulp. It has to be appreciated that the outlines, shapes, and positions of pulp chambers, root canals and pulpal–periradicular foramina are highly variable. Canals may branch, divide, rejoin and present forms that are considerably more involved than many textbooks of anatomy depict for simplicity; yet these simple forms serve to convey the essential canal structure found in teeth. The complex nature of the pulp space is typified by the appearance of an extracted molar rendered transparent (Fig. 1.23).
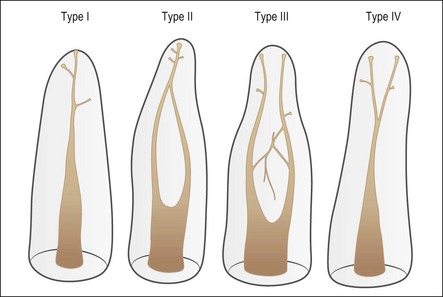
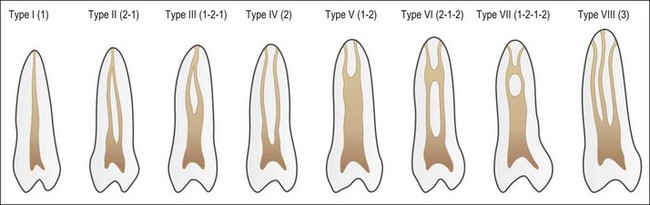
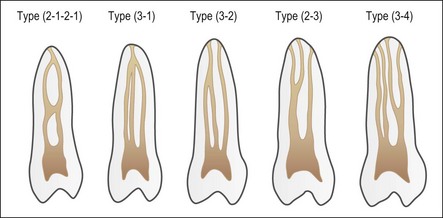
Despite this complexity, it is useful to describe the patterns of root canal system found in human teeth. Few have tried because of the degree of variation evident. Weine et al. (1969) used a four-group classification to describe the canal systems in the MB roots of maxillary molars (Fig. 1.24), which was believed could be applied universally. Zillich & Dowson (1973) used an eight-group classification, which does not appear to have found favour. Vertucci (1984) produced a more complex and extensive classification (Fig. 1.25) to which Gulabivala et al. (2001, 2002) added a number of further groups (Fig. 1.26). These have been more frequently adopted for application in other studies.
Characteristics of the pulp space
In cross-section, root canals tend to take on the shapes of the roots. Where roots are wide in a buccolingual direction, the pulp space tends to take on similar proportions to the outline of the root or there may be more than one root canal (Fig. 1.27).
Roots and root canal systems display simple forms in the mesiodistal dimension, giving a simple radiographic projection; however, this masks great complexity and curvatures in the buccolingual dimension (Fig. 1.28), which is not revealed by conventional radiography. Roots and canals are rarely straight even when they appear so in a normal clinical radiographic projection (Fig.1.29). Cleared specimens further reinforce the complex anatomy (Figs 1.30, 1.31). As a result, the volume of the pulp space is much greater than the normal buccal view might suggest.
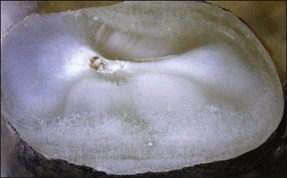
Single roots do not necessarily have single root canals. Even when clinically there appears to be a single opening into a root canal, separation can lead to two distinct canals (Figs 1.32, 1.33). Table 1.5 shows the frequency of two canals in various tooth types. Typically, maxillary anterior teeth possess the type 1 canal system (single central canal following the shape of the root), while the mandibular anterior teeth may possess type 1 or other configurations (types 2, 3, 4 – see Weine classification) as the frequency of two canals is between 40 and 50%. Maxillary first and mandibular second premolars tend to have a type 1 canal system, while the maxillary second and mandibular first premolars can possess more complex systems. The mesiobuccal roots of maxillary molars and the mesial roots of mandibular molars tend to possess complex canal systems (types 2–4 etc), while the palatal and distobuccal roots of maxillary molars tend to possess the type 1 canal. The distal root of mandibular molars may frequently have either a type 1 or type 2 canal system. Characteristics in the form of fins (Fig. 1.34) exist in oval roots with two canals, while cervical bulges (Fig. 1.35) may occur on the palatal aspect of maxillary canine teeth.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses