Chapter 2 The mouth as a microbial habitat
The mouth as a microbial habitat
The properties of the mouth make it ecologically distinct from all other surfaces of the body, and dictate the types of microbe able to persist, so that not all of the microorganisms that enter the mouth are able to colonize. Moreover, distinct habitats exist even within the mouth, each of which will support the growth of a characteristic microbial community because of their particular biological features. Habitats that provide obviously different ecological conditions include mucosal surfaces (such as the lips, cheek, palate, and tongue) and teeth (Table 2.1). The properties of the mouth as a microbial habitat are dynamic, and will change during the life of an individual. During the first few months of life the mouth consists only of mucosal surfaces for microbial colonization. The eruption of teeth provides a unique, hard non-shedding surface which enables much larger masses of microorganisms (dental plaque) to accumulate as biofilms; in addition, gingival crevicular fluid (GCF) is produced which can provide additional nutrients for subgingival microorganisms. The ecology of the mouth will change over time due to the eruption or extraction of teeth, the insertion of orthodontic bands or dentures, and any dental treatment including scaling and restorations. Transient fluctuations in the stability of the oral ecosystem may be induced by the frequency and type of food ingested, variations in saliva flow (for example, certain medications impair saliva flow), and courses of antibiotic therapy.
| Habitat | Comment |
|---|---|
| Lips, cheek, palate |
Mucosal surfaces
The mouth is similar to other ecosystems in the digestive tract in having mucosal surfaces for microbial colonization. The microbial load is relatively low on such surfaces due to desquamation. However, the oral cavity does have specialized surfaces which contribute to the diversity of the microflora at certain sites. The papillary structure of the dorsum of the tongue provides refuge for many microorganisms which would otherwise be removed by mastication and the flow of saliva. Such sites on the tongue can also have a low redox potential (see later), which enable obligately anaerobic bacteria to grow. Indeed, the tongue can act as a reservoir for some of the Gram negative anaerobes that are implicated in the aetiology of periodontal diseases (Ch. 6), and are responsible for malodour (Ch. 4). The mouth also contains keratinized (as in the palate) as well as non-keratinized stratified squamous epithelium which may influence the intra-oral distribution of some microorganisms.
Teeth
Teeth (and dentures) allow the accumulation of large masses of microorganisms (predominantly bacteria) and their extracellular products, termed dental plaque. Plaque is an example of a biofilm (Ch. 5) and, while it is found naturally in health, it is also associated with dental caries and periodontal disease. In disease, there is a shift in the composition of the plaque microflora away from the species that predominate in health (Ch. 6).
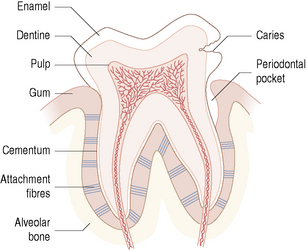
Each tooth is composed of four tissues – pulp, dentine, cementum and enamel (Fig. 2.1). The pulp receives nerve signals and blood supplies from the tissues of the jaw via the roots. Thus the pulp is able to nourish the dentine and act as a sensory organ by detecting pain. Dentine makes up the bulk of the tooth and functions by supporting the enamel and protecting the pulp. Dentine is composed of bundles of collagen filaments surrounded by mineral crystals. Tubules run continuously through the body of the dentine from the pulp to the dentine–enamel and to the dentine–cementum junctions. Enamel is the most highly calcified tissue in the body and is normally the only part of the tooth exposed to the environment. Cementum is a specialized calcified connective tissue that covers and protects the roots of the tooth. Cementum is important for the anchorage of the tooth; embedded in the cementum are the fibres of the periodontal ligament which anchor each tooth to the periodontal bone of the jaw. With ageing, recession of the gingival tissues can expose cementum to microbial colonization and disease (root surface caries; Ch. 6).
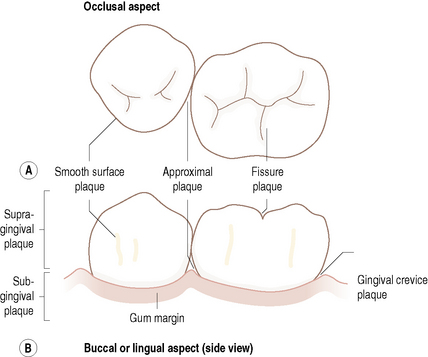
The ecological complexity of the mouth is increased still further by the range of habitats found on the tooth. Teeth do not provide a uniform habitat but possess several distinct surfaces (Table 2.1, Fig. 2.2), each of which is optimal for colonization and growth by different populations of microorganism. This is due to the physical nature of the particular surface and the resulting biological properties of the area. The stagnant areas between adjacent teeth (approximal) and in the gingival crevice afford most protection to colonizing microorganisms from the adverse conditions in the mouth. Both sites are also anaerobic and, in addition, the gingival crevice region is bathed in the nutritionally-rich gingival crevicular fluid (GCF; see later), particularly during inflammation, resulting in these areas supporting a more diverse microbial community. Smooth surfaces are more exposed to the environment and can only be colonized by a limited number of bacterial species adapted to such extreme conditions. The properties of a smooth surface will differ according to whether it faces the cheek (buccal surface) or the inside (lingual surface) of the mouth. Pits and fissures of the biting (occlusal) surfaces of the teeth also offer protection from oral removal forces such as saliva flow, and can contain impacted food debris. Such protected areas are associated with the largest microbial communities and, in general, the most disease.
The relationship between the environment and the microbial community is not unidirectional. Although the properties of the environment dictate which microorganisms can occupy a given site, the metabolism of the microbial community will modify the physical and chemical properties of their surroundings, for example, by consuming oxygen and releasing carbon dioxide and hydrogen to create a more anaerobic environment. Environmental conditions on the tooth also vary in health and disease (Fig. 2.1). For example, as caries progresses, the advancing front of the lesion penetrates the dentine. The nutritional sources will change and local conditions may become acidic and more anaerobic due to the accumulation of products of bacterial metabolism. Similarly, in disease, the gingival crevice develops into a periodontal pocket and the production of GCF is increased. These new environments will select the microbial community most adapted to the prevailing conditions. This is a dynamic relationship, with each change in the local environment invoking a new response by the resident microorganisms, and possibly resulting in a shift in the composition and metabolism of the microflora.
Saliva
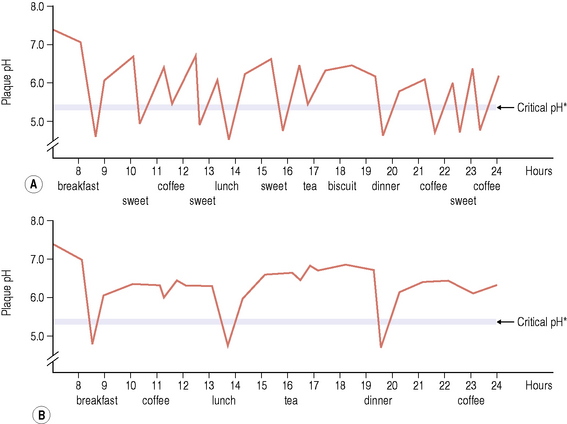
The mouth is kept moist and lubricated by saliva which flows to form a thin film (approximately 0.1 mm deep) over all the internal surfaces of the oral cavity. Saliva enters the oral cavity via ducts from the major paired parotid, submandibular and sublingual glands as well as from the minor glands of the oral mucosa (labial, lingual, buccal and palatal glands) where it is produced. There are differences in the chemical composition of the secretions from each gland, but the complex mixture is termed ‘whole saliva’ (Table 2.2). Saliva plays a major role in maintaining the integrity of teeth by clearing food and by buffering the potentially damaging acids produced by dental plaque following the metabolism of dietary carbohydrates. Bicarbonate is the major buffering system in saliva, but phosphates, peptides and proteins are also involved. The mean pH of saliva is between pH 6.75 and 7.25, although the pH and buffering capacity will vary with the flow rate. Within a mouth, the flow rate and the concentration of components such as proteins, calcium and phosphate have circadian rhythms, with the slowest flow of saliva occurring during sleep. Thus, it is important to avoid consuming sugary foods or drinks before sleeping because the protective functions of saliva are reduced.
Table 2.2 The mean concentration (mg/100 ml) of selected constituents of whole saliva and gingival crevicular fluid (GCF) from humans
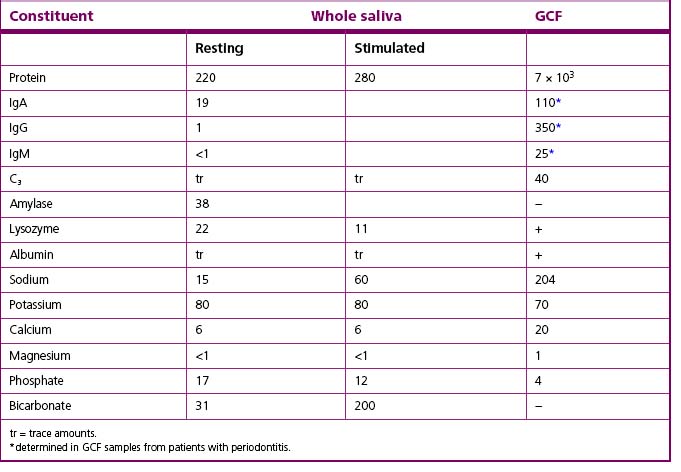
Other nitrogenous compounds provided by saliva include urea and numerous amino acids. Oral microorganisms require amino acids for growth, but not all of these are present free in saliva, and are obtained from salivary proteins and peptides by the action of microbial proteases and peptidases. The concentration of free carbohydrates is low in saliva, and most oral bacteria produce glycosidases to degrade the side-chains of host glycoproteins (Fig. 5.13). The metabolism of amino acids, peptides, proteins and urea can lead to the net production of alkali, which contributes to the rise in pH following acid production after the dietary intake of fermentable carbohydrates (Fig. 2.3).
Antimicrobial factors, including lysozyme, lactoferrin, and the sialoperoxidase system, are present in saliva (Table 2.3) and play a key role in controlling bacterial and fungal colonization of the mouth. Antibodies have been detected, with secretory IgA (sIgA) being the predominant class of immunoglobulin; IgG and IgM are also present but in lower concentrations. A range of peptides with antimicrobial activity, including histidine-rich polypeptides (histatins), cystatins and defensins are also present in saliva. A fuller description of these factors, and a discussion of their role in controlling the resident oral microflora are discussed later in this chapter. The properties of saliva are fundamental to the maintenance of a healthy mouth; consequently, it is often referred to as the ‘defender of the oral cavity’.
Table 2.3 Specific and non-specific host defence factors of the mouth
| Defence factor | Main function |
|---|---|
| Non-specific: | |
| Saliva flow | Physical removal of microorganisms |
| Mucin/agglutinins | Physical removal of microorganisms |
| Lysozyme-protease-anion | Cell lysis |
| Lactoferrin | Iron sequestration |
| Apo-lactoferrin | Cell killing |
| Sialoperoxidase system | Hypothiocyanite production (neutral pH) |
| Hypocyanous acid production (low pH) | |
| Histatins | Antifungal with some antibacterial activity |
| Defensins (α- & β-) | Antimicrobial & immunomodulatory activity |
| Cystatins, SLPI & TIMP | Cysteine, serine & metallo-protease inhibitors |
| Chitinase & chromogranin | Antifungal |
| Cathelicidin | Antimicrobial |
| Calprotectin | Antimicrobial |
| Specific: | |
|---|---|
| Intra-epithelial lymphocytes & Langerhans cells | Cellular barrier to penetrating bacteria and/or antigens |
| sIgA | Prevents microbial adhesion & metabolism |
| IgG, IgA, IgM | Prevent microbial adhesion; opsonins; complement activators |
| Complement | Activates neutrophils |
| Neutrophils/macrophages | Phagocytosis |
Gingival crevicular fluid (GCF)
Serum components can reach the mouth by the flow of a serum-like fluid through the junctional epithelium of the gingivae (Fig. 2.6). The flow of this gingival crevicular fluid (GCF) is relatively slow at healthy sites, but increases by 147% in gingivitis and by up to 30-fold in advanced periodontal diseases, as part of the inflammatory response to the accumulation of plaque around the gingival margin. GCF can influence the microbial ecology of the site in a number of ways. Its flow will remove non-adherent microbial cells, and will also introduce components of the host defences, especially IgG and neutrophils. GCF can also act as an additional and novel source of nutrients for the resident microorganisms (Table 2.2). Many bacteria from subgingival plaque are proteolytic and interact synergistically to break down the host proteins and glycoproteins to provide peptides, amino acids and carbohydrates for growth. Essential cofactors for growth, including haemin for black-pigmented anaerobes, can also be obtained from the degradation of haeme-containing molecules such as transferrin, haemopexin, and haemoglobin.
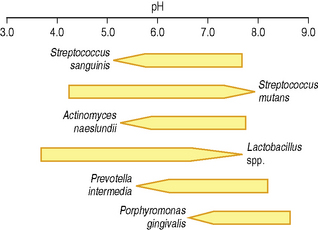
The increased production of gingival crevicular fluid during disease is associated with a rise in the pH of the periodontal pocket. The mean pH during health is approximately 6.90 and this can rise during inflammation in gingivitis and periodontal disease to between pH 7.25 and 7.75. Even such a modest change in pH can alter the competitiveness of individual bacteria, which can affect the proportions of bacteria, especially as some of the putative periodontal pathogens are favoured by an alkaline environment (Fig. 2.4). Also, the activity of some proteases associated with the virulence of these opportunistic pathogens is enhanced at alkaline pH (pH 7.5–8.0).
GCF contains components of the host defences (Tables 2.2 and 2.3) which play an important role in regulating the microflora of the gingival crevice in health and disease. In contrast to saliva, IgG is the predominant immunoglobulin; IgM and IgA are also present, as is complement. GCF contains leukocytes, of which 95% are neutrophils, the remainder being lymphocytes and monocytes. The neutrophils in GCF are viable and can phagocytose bacteria within the crevice. A number of enzymes can be detected in GCF, including collagenase and elastase, which are derived both from phagocytic host cells and subgingival bacteria. These enzymes can degrade host tissues and thereby contribute to the destructive processes associated with periodontal diseases (Ch. 6). Several of these enzymes are under eval/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses