2
The Apical Periodontium
Structure
The periodontium comprises the root cementum, the periodontal ligament, and the alveolar bone. The periodontium attaches the teeth to the bone of the jaws by a fibrous joint, providing a resilient suspensory apparatus resistant to normal functional forces. It should be regarded as an anatomical and functional unit (Fig. 1.9). The apical periodontium is that part of the periodontium which surrounds the root apex.
Composition and Morphology
The cementum and bone are composed of about 65% inorganic material, mostly in the form of hydroxyapatite. The organic matrix consists of 20% collagen and 3% noncollagenous proteins, whereas the remaining 12% is water.
Cementum covers the root surfaces of the teeth. It is avascular, without innervation and does not normally undergo remodeling. Toward the periodontal ligament the cementum is covered by a zone of precementum about 5 mm wide. Bordering the surface, the cementum-forming cells, the cementoblasts, are seen. In the apical periodontium the cementum is cellular with cementocytes in lacunae and canaliculi.
Alveolar bone lines the sockets of the teeth. It is compact and may appear radiographically as a radiopaque narrow zone, the lamina dura. The alveolar bone is perforated by a large number of blood vessels, and osteocytes are embedded in lacunae and canaliculi. Toward the periodontal ligament the bone is covered by unmineralized osteoid and the bone-forming cells, the osteoblasts.
The periodontal ligament consists of dense, unmineralized connective tissue situated between the cementum and the alveolar bone. It is characterized primarily by a large number of collagen fiber bundles that traverse obliquely from the cementum to the alveolar bone. Apically, these fiber bundles radiate outward from the root surface to insert into the surrounding alveolar bone.
Cells, Fibers, and Ground Substance
The apical periodontium contains several cell types. The cementoblasts which form the cementum of the root are highly specialized cells characteristic of the periodontium. Ultrastructurally the cementoblast is similar to the osteoblast and fibroblast of the periodontium and as in the pulp the identity of the various cells is mainly determined by their location.
The major cell type of the periodontium is the fibroblast. The fibroblasts form the fibers and the ground substance of the periodontal ligament. In addition, the collagen fibers undergo almost continuous and rapid remodeling, i. e., formation and degradation. Both of these activities are accomplished by the fibroblasts, sometimes simultaneously. As in the pulp, undifferentiated, probably pluripotential cells are found in the periodontium. These cells are thought to be capable of differentiating into any periodontal cell and are therefore important in repair processes after injury and disease. Mast cells, which may constitute up to 6% of the total cell population of the healthy periodontium, develop from the undifferentiated mesenchymal cells as well.
Macrophages are frequently seen in the healthy periodontium. They have a potential for synthesis of a wide range of proteins which influence other cells. The macrophages are present in many stages of development and show phagocytic activity when stimulated.
The osteoblasts form the alveolar bone. In addition, together with osteoclasts, which are considered normal cells of the periodontium, they are responsible for the continuous remodeling of the alveolar bone.
Also found within the periodontal ligament are clusters of epithelial cells. These derive from the Hertwig’s root sheath which was active during tooth formation. Cell-to-cell junctions are seen between the epithelial cells, and each cluster of cells is surrounded by a basal lamina separating it from the adjacent connective tissue. In addition, the different epithelial cell clusters are connected by delicate processes, allowing them to function as a syncytium. Their primary function is not known, although it has been suggested that they are reponsible for maintaining the periodontal ligament space by preventing the osteoblasts from migrating to and forming bone on the surface of the root. It is clear that the epithelial cells are viable and metabolically active. Under certain pathological conditions they may begin to proliferate and are seen as larger islands or strings of epithelium in inflamed tissue or as the epithelial lining of radicular cysts (see p. 43).
As mentioned above, the fibers of the periodontal ligament are almost exclusively collagenous in nature. However, as in the pulp, a few elastic fibers are associated with the blood vessels. The cells and fibers of the periodontium are embedded in an amorphous ground substance which basically has the same consistency and function as the ground substance of the pulp and connective tissue elsewhere in the body.
Vascular Supply and Innervation
The vascular supply of the periodontal ligament is richly developed (Fig. 1.11). The principal supply of blood is provided by the alveolar arteries. Some branches reach the apical periodontium and course coronally, just prior to entering the pulp, whereas gingival arterioles enter the periodontal ligament and course apically. In addition, numerous vessels enter the ligament through the holes of the alveolar bone. Thus, in contrast to the pulp, the apical periodontium has a rich and well-developed collateral blood supply. A rich capillary network is present as well. It is somewhat more developed near the bone than near the root surface. The lymphatic vessels presumably follow the path of the blood vessels and pass to regional lymph nodes.
The nerves of the periodontal ligament follow the path of the blood vessels as well. Both myelinated and unmyelinated nerve fibers are present, and the sensory innervation is of the trigeminal system. The nerve endings are involved with proprioception and pain perception and they react readily to even slight increases in tissue pressure.
Age Changes
The most obvious age change in the apical periodontium is an increase in the thickness of the root cementum. This tissue is deposited continuously throughout life, and its width will triple between 10 and 70 years of age. In other respects, the remodeling of the periodontal ligament and alveolar bone is highly effective throughout life, and these components of the periodontium will be modified more according to external influence than to age. Still, a decrease in the rate of collagen synthesis will occur as well as certain changes in the ground substance. It also appears that tissue repair after inflammation of the apical periodontium in elderly patients may occur by the formation of an unmineralized fibrous tissue rather than the formation of new bone (see p. 52).
Reaction Patterns
Apical Periodontitis
The pulp and the soft tissue of the apical periodontium constitute a continuum via the apical foramina. Untreated pulpal inflammation will, therefore, gradually spread beyond the apex of the tooth. This condition is termed periradicular, peri-apical, or most commonly apical periodontitis. If the inflammation occurs on the side of the root adjacent to a lateral root canal, the term lateral periodontitis may be used. At first only the periodontal ligament will be involved in the periapical reaction. However, soon there will be resorption of cementum (and dentin) and alveolar bone so that all tissues of the periodontium will be affected. Thus, apical periodontitis is a pulpally related inflammation of the attachment apparatus of the tooth. The inflammatory process may lead to considerable bone loss, sometimes encompassing large areas of the alveolar process. Quite commonly a fistulous tract develops from the inflamed area to a body surface, in most instances to the oral vestibule.
Etiology of Apical Periodontitis
Pulpitis
A local pulpitis in the coronal pulp may occasionally cause inflammatory changes in the apical periodontium. This is recognized clinically by the fact that the tooth becomes tender to biting or percussion. As discussed above, pulpal inflammation leads to vasodilation and an increase in capillary permeability with filtration of fluids from the blood vessels into the tissue. In most instances lymphatics and blood vessels of the uninflamed areas of the pulp can deal with the increased tissue fluids and transport the fluids away. However, when periapical symptoms arise, this mechanism is apparently flawed or insufficient, and an increase in tissue pressure in the apical periodontium occurs, leading to tenderness of the tooth. Sometimes the tooth is pushed out of its alveolus so that a widened periodontal ligament space appears radiographically. When the increased tissue pressure in the pulp is relieved, either spontaneously or as a result of treatment, the periapical symptoms will disappear as well.
Pulp Necrosis
In teeth with necrotic pulps, the content of the root canal will be dead cells, stagnated tissue fluids, and tissue breakdown products. Many of these tissue components will have a cytotoxic effect and traditionally such breakdown products have been regarded as the main etiological factors for apical periodontitis. However, it is now established that as long as no nonself proteins or other foreign substances are present in the root canal, the peri-apical response is quite mild, and will not lead to bone resorption and the formation of an apical granuloma (Fig. 2.1). The reason for this is that necrotic tissue as such causes only a mild chemotactic activity in serum and has only a slight immunogenic potential. Clinically, the benign nature of the reaction is recognized by the fact that apical periodontitis is not evident radiographically as long as the necrotic tissue of the root canal is not infected with microorganisms.
Infection of the Root Canal
It has been known for a century that bacteria may colonize the root canal. The importance of bacteria as an etiological factor for pulpal and periapical inflammation as expressed in the literature has varied over the years. However, striking evidence for the role of infection came in experiments in the 1960 s when it was shown that pulp necrosis and apical periodontitis would not develop in germ-free animals when the pulp was exposed to the oral cavity. In humans it has been shown that an apical periodontitis with bone resorption will develop only if the necrotic pulp becomes infected. Finally, it is known that bacteria which are isolated from root canals of teeth with apical periodontitis will cause an apical periodontitis when inoculated in the root canal of other teeth. When these bacteria are reisolated, it has been shown that they in fact are the inoculated organisms and therefore have the capacity to establish themselves and survive in the root canal and exert pathological influence on periapical tissues.
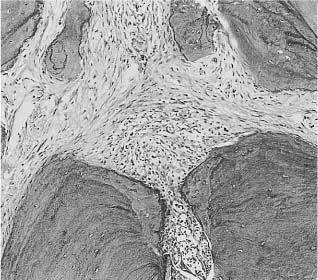
Fig. 2.1 Root tip of a monkey tooth. Pulpal inflammation has reached the apical foramen and involves a small area of the periodontal ligament (hematoxylin-eosin).
If a root canal is exposed to the mouth, bacteria will accumulate in the pulp chamber and the canal. The normal plaque/saliva microflora dominated by facultative anaerobic organisms will be present in the root canal. Many strains of obligate anaerobic bacteria will be present as well, but usually in small numbers. If the root canal has a wide opening to the mouth, periapical inflammation with bone destruction will not develop in a predictable manner. However, if the access opening to the root canal is sealed off after the oral flora has been allowed to colonize the root canal system, a selective bacterial growth begins. Already after 7 days, 50% of the flora is anaerobic bacteria, and soon some 90% of the bacteria may be anaerobic. In the apical area of the root canal where the oxygen tension is lowest, the predominance of anaerobic bacteria is even greater than in the main canal. From the root canal the bacteria may enter the tubules of the root dentin (Fig. 2.2). One must therefore understand that the term root canal infection, which is commonly used, in reality means infection of the root canal system with the main canal, side canals, and apical deltas, as well as infection of the root dentin.
Fig. 2.2 Biofilm on root canal wall in nonvital tooth. Bacteria from the root canal have entered the underlying dentinal tubules (Brown–Brenn stain).
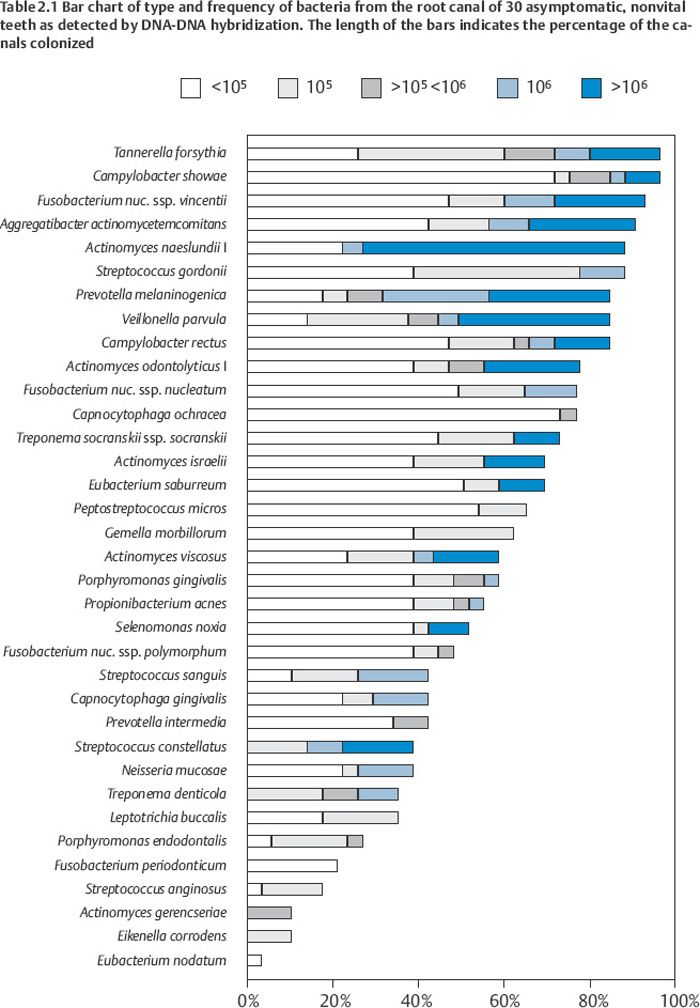
Although it has been estimated that the oral flora consists of more than 700 different bacterial species, usually less than 10 cultivable species are recovered from the root canal. However, more than 50% of the oral flora is uncultivable, and only recently have molecular techniques made it possible to obtain a more accurate detection of bacteria and bacterial DNA from clinical samples. Thus, in a recent study, the root canal of 30 teeth with asymptomatic apical periodontitis was evaluated for the presence of 40 commonly occurring micro-organisms in endodontic and periodontal infections using whole genomic DNA probes and DNADNA hybridization. The DNA of 35 bacteria was detected with a range of 5–31 species (Table 2.1). Five probes were negative for all root canals. For the sake of comparison, bacterial samples from the root canal of the same teeth were cultured aerobically and anaerobically and 42 microorganisms with the “normal” range of 0–6 species per canal were recovered. Clearly, there will have to be more uncultivable bacteria than were uncovered with our 40 probes. Thus, a reasonable estimate at present might be that an infected root canal contains, not less than 10, but rather between 10 and 50 bacterial species. Interestingly, this coincides well with the number of microorganisms found in biofilms in the healthy oral cavity and subgingivally in patients with active periodontal disease.
Considering the large number of microorganisms in the oral cavity, the microbial composition of the root canal flora will have to vary. However, given the results of the molecular study mentioned above it becomes clear that the microbiota of the root canal is very similar to the microbiota of the periodontal pocket in patients with active periodontal disease. Thus, Tannerella forsythia, Campylobacter showae, Fusobacterium nucleatum ssp. vincentii, and Aggregatibacter actinomycetemcomitans were present in more than 90% of the root canals. Other designated periodontopathogens like Porphyromonas gingivalis (60%), Campylobacter rectus (80%), Prevotella intermedia (50%), Selenomonas noxia (60%), Peptostreptococcus micros (70%), Treponema socranskii (70%), and Treponema denticola (40%) were commonly present as well. The “red complex” bacteria T. forsythia, P. gingivalis, and T. denticola, which are known to have a decisive influence on the progression of disease in patients with active marginal periodontitis, were together present in 40% of the root canals.
Porphyromonas endodontalis, which in combination with other root canal bacteria is known to cause transmissible infection in guinea pigs, was recovered from 30% of the canals. Interestingly, combinations of the same endodontic bacteria, but without P. endodontalis, did not cause transmissible infection in the same experiments. Still, these and other noninfective bacteria may play an important role in maintaining the root canal infection by providing growth factors for the principal pathogens and by synthesizing and degrading extracellular materials in the biofilm.
The associations of bacteria in a mixed infection is not random. With regard to oral bacteria, this is best known from studies on dental plaque where six closely associated groups or complexes of bacterial species are recognized. The red complex mentioned above is one of the groups. An “orange” complex including Campylobacter rectus, C. showae, Eubacterium nodatum, Fusobacterium nucleatum, P. intermedia, P. micros, and P. nigrescens is important for disease progression as well. A third group includes the Actinomyces and a fourth group consists of Capnocytophaga species, A. actinomycetemcomitans, Eikenella corrodens, and Campylobacter concisus. The streptococci make up the fifth group and the sixth group comprises Veillonella parvula and Actinomyces odontolyticus.
Clearly, the root canal is different from the periodontal pocket, and at present it is not known whether the findings in plaque are totally valid in endodontic infections. However, in established root canal infections where Gram-negative species are numerically important, Fusobacterium nucleatum plays a dominating role. This species congregates with most oral bacteria, including strains of P. gingivalis, T. denticola, A. actinomycetemcomitans, P. intermedia, Eubacterium species, Selenomonas species, and Actinomyces species. The important red and orange complexes contain most of these coaggregating species.
Microorganisms are also frequently found in the root canal of root filled teeth with apical periodontitis, i. e., in root filled teeth where the treatment has failed. However, in these teeth the flora is different from the flora of untreated non-vital teeth in that it is heavily dominated by facultative anaerobic species. Enterococci, streptococci, and staphylococci are frequently isolated organisms under these conditions. Clinically, this is important in that a different antimicrobial regime might be called for in the retreatment of the failed cases than would normally be used in primary treatment of root canal infections.
Portal of Entry of Bacteria
The crown of the tooth is the main portal of entry for bacteria into the pulp and root canal space. This is readily understood in teeth with pulp exposures or in teeth with carious lesions. At first it may be somewhat more difficult to see how the bacteria can reach the pulp through an intact crown. However, in reality there is rarely such a thing as an “intact” crown. Teeth have dentin exposed by attrition or abrasion, or by scaling and root planing during prophylaxis and treatment of periodontal disease. Enamel–dentin cracks filled with plaque and bacteria are commonly present in teeth. They are almost bound to run at an angle with the dentinal tubules so that a single crack may lead to exposure of a large number of tubules (Fig. 1.30).
Another possible pathway of infection of the necrotic pulp and root canal space is the apical foramina and accessory canals through a hematogenous spreading of the bacteria. However, the evidence for this is presently inconclusive. Still, it is known that oral bacteria that readily enter the bloodstream following dental treatment and prophylactic measures may settle down at sites where there is tissue damage, conceivably also in necrotic tissue of the root canal. The fact that the bacteria isolated from the root canal are also found in the periodontal pocket has been regarded as a strong indication that a hematogenous spreading of bacteria to the root canal indeed occurs. However, exposed dentin is especially prevalent in the cervical area of the teeth adjacent to the periodontal pocket, and infection of the pulp by periodontal bacteria via a direct route through the dentinal tubules seems entirely possible (Fig. 2.2).
Infection of the Periapical Lesion
Traditionally it has been held that in teeth with asymptomatic apical periodontitis the microorganisms are in biofilms in the root canal system and in tubules of the root dentin, whereas the periapical lesion is free of bacteria. Probably, the defense systems mobilized by periapical inflammation at first will eliminate the bacteria from the root canal that invade the periapex. However, in long-standing infections with a more or less permanently established microflora in the root canal, the host defenses are less effective, and microbial invasion of the periapical lesion may take place.The evidence for this comes from studies of surgically removed periapical lesions using traditional culturing as well as molecular microbiological techniques.
Of interest in this regard are also recent findings suggesting the involvement of herpes viruses in the etiopathogenesis of apical periodontitis. The viruses may cause the release of tissue-destructive cytokines and the initiation of cytotoxic and immunopathologic events. The immune impairment and tissue changes resulting from the virus infection may then aid bacteria in invading and surviving in the periapical lesion.
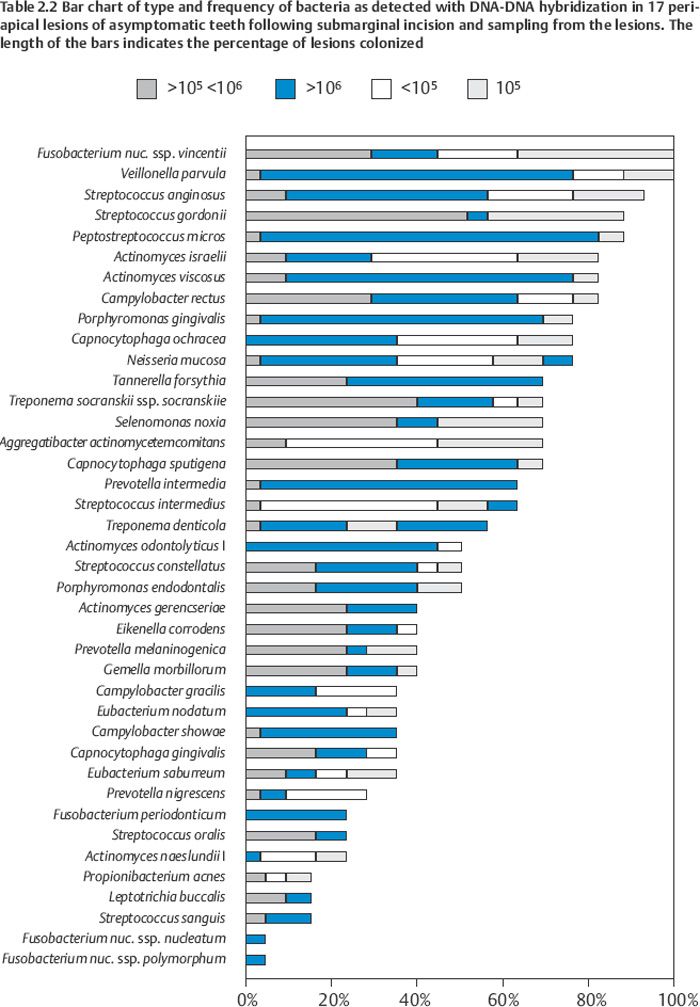
As in the root canal the infection of the periapical lesion is characterized by a wide variety of combinations of bacteria. Some 20–30 bacterial species are recovered from a granuloma and about half to two thirds are anaerobes (Table 2.2). As in the root canal Fusobacterium, Porphyromonas, Prevotella, Campylobacter, and Treponema species are commonly isolated. In one study, the “red complex” bacteria Tannerella forsythia, Porphyromonas gingivalis, and Treponema denticola were present in 70% of the lesions and Porphyromonas endodontalis was present in 50%. Of Gram-positive anaerobes, Actinomyces, Propionibacterium, Peptostreptococcus, and Eubacterium species are frequently present. In addition, facultative Actinomyces, Streptococcus, Enterococcus, and Staphylococcus species are recovered.
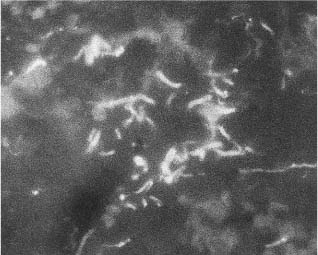
Recently, a fluorescence in-situ hybridization method has been developed whereby bacteria may be observed and even identified in the tissue of their natural environment. With this technique it has been possible to visualize bacteria directly within periapical lesions of asymptomatic root-filled teeth (Fig. 2.3). The bacteria were present in localized areas of the lesions, whereas other areas were free of bacteria. A variety of bacterial morphotypes were seen: cocci, rods, spiral- and spindle-shaped. Organisms with different morphologies were seen to coaggregate forming small ecosystems, possibly biofilm in the tissue. In some areas looser groups of bacteria were present, and single organisms, especially spirochete-like bacteria were seen spread out among fibers and cells. Motility and chemotaxis are major virulence factors of these organisms and they are highly invasive in host tissue. Oral treponemes have been shown to carry several putative virulence factors identical or similar to invasive organisms such as Treponema pallidum, the causative organism of syphilis. Probably the importance of treponemes in endodontic infections is highly underestimated.
In periapical lesions refractory to endodontic therapy, the periapical flora is highly dominated (80%) by Gram-positive organisms and is clearly different from the root canal flora that normally responds to treatment. In one study, 75% of the refractory lesions contained Streptococcus, Staphylococcus, Bacillus, Pseudomonas, Stenotrophomonas, Sphingomonas, Enterococcus, Enterobacter, or Candid a species, and typically the organisms persisted in five of eight patients who had taken antibiotics systemically before sampling. Several of these microorganisms have adapted over time to live in many different environments. They may be surrounded by extracellular materials in a biofilm-like structure (Fig. 6.15), or the bacterial aggregates may have the form of biofilm granules with diameters up to 3–4 mm (Fig. 2.4). These granules often have a bright, yellow color, and because of this in older literature are referred to as sulfur granules. One or more Actinomyces species is as a rule recognized in the granules, but in addition a wide spectrum of other bacteria like Propionibacterium, Staphylococcus, Peptostreptococcus, Pseudomonas, and Treponema species are recovered from the granules. Most of the organisms in the biofilm granules are simultaneously present in the periapical lesions.
Fig. 2.3 Section from periapical endodontic lesion. Clinical diagnosis: Asymptomatic apical periodontitis. A probe specific for the domain Bacteria, EUB 338, has been used to visualize the bacteria in the granulation tissue.
In cases where the endodontic treatment has been incorrect or grossly inadequate, for instance, after repeated but inadequate antibiotic treatment, often in conjunction with multiple openings and closings of the root canal or incorrect and unsuccessful peri-apical surgical treatment, the presence of enteric and environmental bacteria and yeast is especially conspicuous. In such instances organisms like Escherichia coli, Bacteroides fragilis, Pseudomonas aeruginosa, Enterobacter, Clostridium, Proteus, and Klebsiella species and yeast are recovered. The presence of these and other mainly nonoral microorganisms suggest that blood-born infection of the periapical lesion may have taken place.
As appears from the above, endodontic infections are polymicrobial in nature and, interestingly, the flora is very similar to the flora of the periodontal pocket in patients with active periodontal disease. The red and orange complexes of bacteria regarded as important for the progression of periodontal disease apparently are important pathogens also in endodontic infections. No single pathogen of special importance has been identified. Still, the role of Actinomyces needs to be discussed. Traditionally, Actinomyces species, especially A. israelii, have been regarded as specific pathogens causing the infectious disease Actinomycosis in a similar manner to Treponema pallidum causing syphilis and Mycobacterium tuberculosis causing tuberculosis. This may be incorrect. In periodontal research it has been shown that Actinomyces are important colonizers and scaffold builders in dental plaque both in health and disease, but also that there is a significant decrease in the Actinomyces species and an increase in the proportion of members of the red and orange complexes in patients with active periodontal disease. In the biofilm granules observed in periapical lesions, Actinomyces species are never isolated as the sole organism, but with other known pathogens normally found in endodontic and periodontal infections. Thus, so-called actinomycotic infections appear to be polymicrobial in nature. It is well established that Actinomyces may survive under harsh conditions and that they have special surface structures enabling them to attach to a variety of surfaces. Thus, their role may well be to create such conditions that other bacteria (the real pathogens) are attracted to the site and may establish themselves there.
Fig. 2.4
a Surgical treatment of refractory apical periodontitis.
b Three granules of diameters between 2 and 4 mm of a bright yellow color (so-called sulfur granules) are recovered from the periapical lesion.
c Scanning electron micrograph of surface area of sulfur granule seen in b. Microorganisms that are tightly packed and glued together make up the outer boundary of the granule. Two macrophages are seen engulfing bacteria.
d Cut surface of granule showing an abundance of bacteria. Rod-like organisms are prominent and spiral-formed bacteria can be seen.
e From cut surface of granule. In addition to spiral-formed and rod-like bacteria, an amorphous material can be seen.
Iatrogenic Factors
Periapical inflammation may be caused by iatrogenic factors, i. e., instruments, medicaments, and materials used in endodontic treatment. However, bacteria may be present in the root canal or may be introduced inadvertantly during endodontic treatment, and there is little doubt that many clinical reactions that in the past have been ascribed to iatrogenic factors are in fact due to infection.
Still it is clear that when a pulp is extirpated, a soft-tissue wound is created near the apical foramen of the tooth. The wound, which represents tissue injury, will cause an inflammatory response as the first phase of tissue repair. If no further irritants are present, repair will occur in 3–4 weeks. The severity of the tissue response to instrumentation will depend on the degree of tissue injury, and vigorous over-instrumentation with laceration and removal of periapical tissue may cause an acute apical periodontitis, sometimes with slight bone resorption so that a periapical radiolucency may be seen. However, in the absence of bacteria, repair will occur uneventfully. In the presence of bacteria, on the other hand, over-instrumentation is an effective way to spread the infection. The contaminated endodontic instruments will cause puncture wounds in the periapical tissues, and the bacteria readily survive and multiply in the damaged and dead tissue. Under such conditions over-instrumentation may lead to proliferation of epithelial cells in the periodontal ligament and possibly to apical cyst formation.
Antiseptics are used in the root canal for irrigation during instrumentation and as intracanal medicaments to obtain a bacteria-free canal. By definition these medicaments have an antibacterial effect, but they also have a toxic effect on human cells. All the antiseptics used in endodontics, especially phenolic and aldehyde compounds, are potent tissue irritants (Fig. 2.5). Thus, the intracanal medicaments may cause periapical cell death. Clinically, this is recognized as exudation from the periapex into the root canal. Sometimes it may be difficult to distinguish between exudate from an infectious and a chemical inflammation. However, if no bacteria are present, discontinuation in the use of the cytotoxic medicament will result in rapid repair.
Possibly all root canal sealers which are used today have a certain tissue-irritating effect, especially in the freshly mixed state. However, if a sealer is confined to the root canal space, the tissue–material interface is small and the inflammatory reaction in the contacting tissue will be mild. After a few weeks when the setting reaction of the materials is completed, the materials generally have no further biological effect, and in the absence of bacteria, tissue repair will occur. If the root canal is overfilled so that material is forced into the periapical tissues, the tissue–material interface is larger and the tissue irritation more severe. Also, when a material is forced into a tissue, it will have a rough, uneven surface that will cause tissue irritation and inflammation mechanically. Thus, a radiolucent zone may develop surrounding a material in the periapical tissues. Macrophages will be activated and gradually the uneven surface of the material will become smooth. A fibrous capsule will then form around the material and the repair process is completed. However, depending on the type and consistency of the material, it may also be completely removed, either by phagocytes, by dissolution in the tissue fluids, or by both.
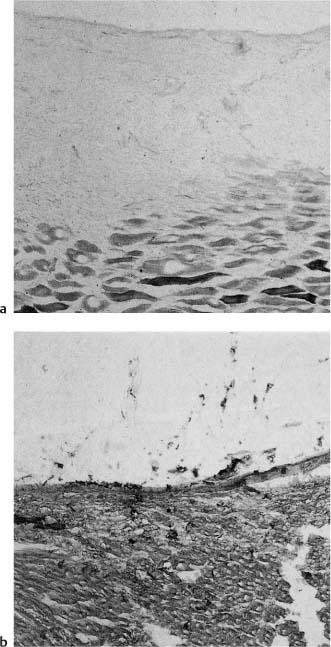
Fig. 2.5
a Nonepithelialized tissue exposed to 10% formalin for 15 min incubated for the demonstration of succinate dehydrogenase. No oxidative enzyme activity is evident in the surface area of the affected tissue, demonstrating the cytotoxic effect of formalin.
b Control section not exposed to formalin showing oxidative enzyme activity to the surface.
Pathogenesis of Apical Periodontitis
Action of Bacteria
Apical periodontitis may be acute and symptomatic or chronic and asymptomatic. A chronic course/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses