10
Endodontic Materials
Endodontic materials in the widest sense are:
1) Cavity base materials with a therapeutic effect,
2) Pulp capping agents,
3) Materials used for temporary closure of access cavities in teeth during endodontic treatment,
4) Root canal filling materials, and
5) Materials used for retrograde filling of the root canal, repair of root perforations, etc.
Of these, base materials and pulp capping agents have been discussed above (see p. 86 and p. 88).
Temporary Filling Materials
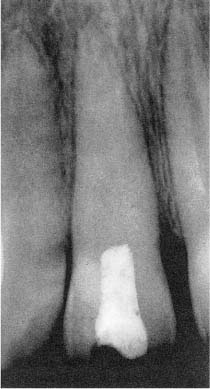
An absolute requirement of a material for temporary closure of an access cavity is that it provide a bacteria-tight seal. In other words, the material must prevent oral bacteria from reaching the un-filled root canal. Most widely used for this purpose is a paste of zinc oxide and eugenol. Such a paste gives a good seal and has an antibacterial effect which adds to its protective properties. The thickness of the temporary filling is another factor which contributes to its sealing ability, and a zinc oxide–eugenol-type filling should be at least 3–4 mm and if at all possible 5 mm thick (Fig. 10.1). The root canal will then be well protected for several weeks. If longer periods of protection are required, a resin-reinforced zinc oxide–eugenol material like IRM cement may be used.
Eugenol is a substance that inhibits the polymerization reaction of resins, and can therefore interfere with the bonding of a final composite resin restoration in the access cavity. However, if the right precautions are taken, the influence of eugenol on the cavity walls can effectively be eliminated. The dentin should be cleaned mechanically with small burs and then washed with alcohol. A normal acid-etch and rinse technique may then be used, resulting in a resin–dentin bond as strong and reliable as if a eugenol temporary cement had not been used.
A temporary filling material, Cavit, which is based on zinc oxide and calcium sulfate, is another alternative. Cavit is available in tubes and jars ready-mixed to be inserted into the access cavity. The material will set under the influence of moisture (saliva) and it gives a reliable bacteria-tight seal when used in the necessary thickness (3–5 mm).
Fig. 10.1 Radiograph of a maxillary incisor with a zinc oxide–eugenol temporary filling. The filling seals the access cavity as well as the pulpal ends of possibly exposed dentinal tubules in the cervical area of the tooth.
Filling materials like amalgams, resins, and most cements will not give an adequate bacteria-tight seal of an access cavity. If it is desirable that such materials be used between endodontic visits for strength or esthetic reasons, the orifice of the root canal has to be sealed bacteria-tight first before the restorative material is inserted.
Root Canal Filling Materials
Root canal filling materials are protected by the dentin wall of the root canal and by occlusal or incisal restorations. However, the materials are in close contact with soft connective tissue apically in the root canal so that tissue–material interactions occur. The root canal filling, therefore, is comparable to an implant elsewhere in the body.
Ideally, a root canal filling material should permanently seal the root canal bacteria-tight. It should not irritate the apical tissues and it should not dissolve or disintegrate as a result of influences from tissues or tissue fluids. Also, a material used in the root canal should be radiopaque so that it can be determined radiographically whether a tooth has been treated endodontically or not. Moreover, radiographic examination is the only method by which the apical extent of a root canal filling and, very importantly, its technical quality can be determined. A root canal filling material should also be easily removed from the canal, as retreatment of teeth often becomes necessary because of treatment failure and because root canals are frequently used for retention of posts in the restoration of endodontically treated teeth.
A great number of materials and material modifications have been used as root canal filling materials, but there is still no material available today which fulfills all of the above-mentioned criteria. As a consequence, a combination of materials is most often used to fill the root canal. A tissue-compatible core material which can readily be removed from the canal but which does not give the necessary seal is used to fill the bulk of the root canal space. In addition, small quantities of a paste-like material that provides a bacteria-tight seal is used to fill minor spaces between the core material and the root canal wall. Thus, root canal filling materials may be divided into two distinct groups: core materials and sealing materials, or root canal sealers.
Core Materials
There are two types of core materials in common use, gutta-percha and a resin-based material (Resilon). In addition, points of metallic silver were widely used to fill root canals and may still be used in some areas.
Gutta-percha. Gutta-percha is a polymer, mainly polyisoprene, which is extracted from a tropical tree in Malaysia. At room temperature it is 60% crystalline, while the rest of the mass has an amorphous structure. As is normal for polymers, the material is viscoelastic, which means that it has a certain elasticity, but at the same time it has the characteristics of a viscous fluid. When heated, gutta-percha is softened and deformed and it becomes liquid when the temperature exceeds 65° C. The material can also be dissolved in organic solvents such as chloroform, xylene, and eucalyptol. If gutta-percha is exposed for some time to light and air, it oxidizes and becomes hard and brittle. It can be reconditioned by means of warm water (40° C).
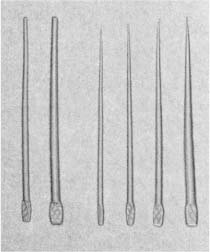
Already at the beginning of the 19th century, attempts were made to use gutta-percha in dentistry, but without success. Not until it was learned how to alter its physical characteristics by adding zinc oxide and other substances did it receive real attention. It has been in continuous use as a root canal filling material since the 1860s and is still by far the most widely used material for obturation of the root canal space. Today, gutta-percha is available in two types of point, standardized points and accessory points (Fig. 10.2). The composition of the points may vary from one manufacturer to the other, but generally they contain 60–70% zinc oxide, up to 17% heavy metal salts, and 1–4% waxes, resins, antioxidizing agents, etc. The actual amount of gutta-percha in the points, therefore, is only about 20% of the total content.
Fig. 10.2 Gutta-percha points for root canal filling. Standardized points nos. 50 and 80 (left); accessory points of the sizes A, B, C, and D (right).
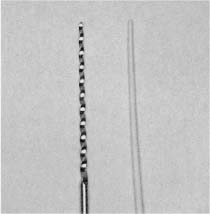
The standardized gutta-percha points are manufactured to correspond in size and shape to the standardized root canal instruments (Fig. 10.3). However, due to the nature of the material, the manufacturing process of standardized sizes is difficult, and diameter tolerances of ±0.05 mm have to be accepted at the present time. This is unfortunate, as two points of allegedly the same size may vary in diameter as much as 0.10 mm, which in points smaller than no. 60 may represent 3 instrument sizes. Still, even with its present weaknesses, the standardization of the gutta-percha points is extremely helpful when selecting a master point to fit as well as possible in the apical part of the root canal. In addition to the standardized master points with taper 0.02 mm, standardized points with tapers 0.04 mm and 0.06 mm are also available.
The accessory gutta-percha points have a pointed shape (Fig. 10.2). They are available in assorted sizes and are used to supplement the standardized master point to fill the coronal flared part of the root canal.
Gutta-percha points fulfill most of the requirements of a root canal filling material. An important characteristic of the material is its favorable biological properties in that it is virtually nonirritating to contacting connective tissue. Moreover, with a sensible technique, it is relatively easily inserted into the root canal. It does not discolor the tooth, it provides good radiographic contrast, and it is readily removed from the canal. However, gutta-percha does not provide a bacteria-tight seal of the root canal. Attempts have been made to overcome this weakness by softening the points in an organic solvent, usually chloroform. In this manner, the material can be molded according to the shape of the canal to fill it completely in three dimensions. However, the material loses its dimensional stability when exposed to a solvent, and when the solvent has evaporated, shrinkage will occur. Gutta-percha will shrink if softened by heat as well and regardless of method, the more it is softened, the larger is its shrinking potential. Thus, gutta-percha points should preferably not be softened at all, but even if they are, they should always be used in combination with a second material, a root canal sealer, to obtain the necessary bacteria-tight seal of the root canal.
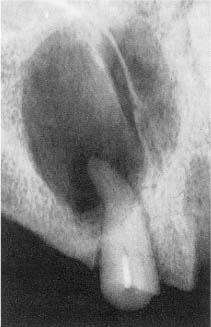
Silver points. Metals have been used as root canal filling materials for thousands of years (Fig. 10.4). Gold, silver, and lead were especially popular due to the relative softness and ductility of these metals. Historically, the largest area of use was the filling of extracted teeth which were to be transplanted from one individual to another.
Since the 1920s, metallic silver points have been used as root canal filling material (Fig. 10.5). One reason silver was preferred to other metals was its alleged oligodynamic effect, i. e., the silver points were believed to release silver ions with a bactericidal effect due to their affinity for certain bacterial enzymes. Moreover, silver is relatively soft, which permits adaptation of a straight point in curved canals.
Fig. 10.3 Standardized root canal instrument (Kreamer) and standardized gutta-percha point. The standardized points correspond in size and shape to the standardized instruments.
Fig. 10.4 Radiograph of a maxillary incisor with a metallic root canal filling in an ancient man (approximately 200 B.C.). Note that a large radiolucent periapical lesion has developed.
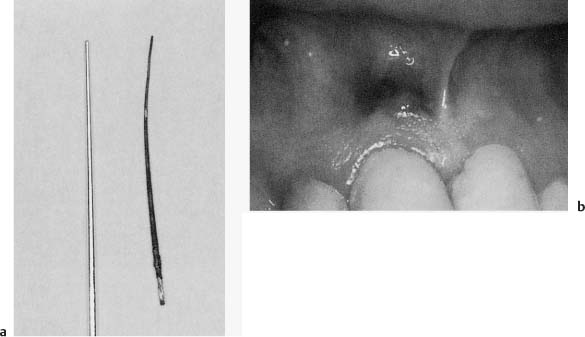
Today, we know that silver ions in fact do have an antibacterial effect, but also that they are not released spontaneously from metallic silver. On the contrary, pure metallic silver is nontoxic and nonirritating to human cells as well as to bacteria. However, when silver has been in contact with tissue and tissue fluids for some time, as, for example, in the root canal, it corrodes (Fig. 10.6). Among the corrosion products are silver–sulfur compounds and others which are extremely toxic and may cause inflammation in adjacent tissues. For this reason silver points are used less and less in endodontic therapy and should preferably not be used at all.
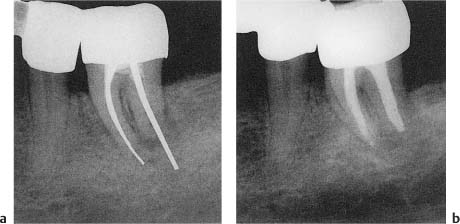
Fig. 10.5
a Radiograph of a mandibular molar with silver point root canal fillings. The case is failing and apical radiolucencies and root resorption are evident.
b Periapical repair after the removal of silver points, retreatment of the tooth and obturation of the root canals with gutta-percha.
Fig. 10.6
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses