Systemic Factors Influencing Dental Implant Therapy
Steven J. Sadowsky
University of the Pacific Arthur A. Dugoni School of Dentistry, San Francisco, California, USA
Despite the fact that only a small percentage of adult patients are precluded from dental implant therapy because of absolute contraindications, surgical placement in these individuals may have serious sequelae, including possible implant failure, refractory healing, and even life‐threatening consequences. The American Society of Anesthesiologists (ASA) has developed a classification system to stratify patients’ physical status in order to assess relative risk for surgical procedures (Table 2.1). Maloney et al.1 have recommended that elective treatment (implant placement) should be reserved for classification I–III. While this can serve as a general guide, implant treatment may run the gamut from simple and minimally invasive to most complicated.2 Moreover, the degree of systemic disease control can be labile and subtle. Finally, the influence of specific disease entities or medications on dental implant survival is unclear in the literature as there are few randomized controlled trials, if any, evaluating health status as a risk factor.3
Table 2.1 ASA classification system for assessing relative risk for surgical procedures.
| ASA I Healthy |
ASA II Mild systemic disease |
ASA III Severe/not incapacitating disease |
ASA IV Incapacitating |
ASA V Dying |
|
| Walk up two flights of stairs | Yes | Rest at completion | Rest before finishing | Unable | Unable |
| Examples | Healthy | Drug allergy 140–159 systolic 90–94 diastolic Controlled diabetic Controlled asthmatic Controlled epilepsy Controlled asthma |
Stable angina pectoris 160–199 systolic 90–114 diastolic Chronic obstructive pulmonary disease Myocardial infarction (MI) >6 months prior Stroke more >6 months prior |
Unstable angina MI <6 months ago >200 systolic >115 diastolic Severe heart failure Uncontrolled diabetes |
End‐stage disease |
| Treatment modification | None | Stress reduction protocols where needed | Elective care OK Stress reduction recommended |
Noninvasive only | Palliative only |
Suggested absolute contraindications to implant placement include profound immunosuppression, uncontrolled diabetes, intravenous bisphosphonates, bleeding disorders, recent myocardial infarction/stroke, active treatment of malignancy, alcohol/drug abuse, and neuropsychiatric illness. Commonly proposed relative contraindications include osteoporosis, smoking, chronic periodontitis, autoimmune disease (nondiabetic conditions), and lack of surgical experience, but there is weak evidence to support recommended absolute or relative contraindiactions.4 Temporary contraindications include incomplete growth, pregnancy, and acute infection. A discussion of these potential risk factors will be helpful to assess the best strategy in orchestrating implant therapy, considering the benefit/risk calculus.
Immunocompromised patients
Immunocompromise can diminish the patient’s ability to battle infection following implant surgery. There is not a robust body of evidence‐based literature to determine the influence of immunosuppression on implant survival.
Case reports and a case series have demonstrated implant survival up to and including more than 5 years’ follow‐up on patients with organ transplants.5,6 Despite the fact that long‐term cyclosporin A usage has been shown to impair initial peri‐implant bone healing and osseointegration in animals, patients receiving liver or kidney transplants and concomitant immunosuppressant therapy have had unremarkable implant outcomes.7 However, given the low‐level evidence and small population size, caution should be used when implants are placed in these patients.
The human immunodeficiency virus (HIV+) has a significant impact on a patient’s immune function. The use of highly antiretroviral therapy (HAART) has extended the life‐span of these patients and qualified them for implant treatment.
Patients infected with the HIV+ been reported in the short term to have similar outcome measurements to HIV‐ patients after activation of implants in a mandibular two‐implant overdenture opposing a complete denture scenario.8 These included 20 patients with a CD4 (glycoprotein on surface of T‐helper cells) count ranging from 132–948/mL. Another short‐term study substantiated that implant treatment in HIV+ patients proved to be a predictable approach in posterior mandibles.9 Given the absence of published long‐term success, it is propitious to place implants in HIV+ patients when CD4 rates are greater than 250/mL, the viral load is below 50/mL, and the patient is on antiretroviral therapy.10 Optimized oral hygiene regimens, regular recall appointments, and screening for HIV‐related oral lesions and xerostomia are recommended to treat the side effects of antiretroviral therapy.
Systemic corticosteroids can cause suppression of the hypothalamus–pituitary–adrenal axis and patients on systemic steroids are therefore at risk of adrenal insufficiency when undergoing implant surgery. Patients who have completed a short course of this therapy (less than 3 weeks) may be at risk and it is recommended that they be given steroid coverage.3 On the other hand, a history of recent prescribed doses of less than 10 mg prednisone daily can be treated without additional steroid therapy.11 Aside from the fact that medical advice should be garnered for immunosuppressed patients and infection‐control measures must be strictly enforced, systemic corticosteroid treatment is not an absolute contraindication to implant placement.
Uncontrolled diabetes
Diabetes mellitus is a chronic disorder of carbohydrate metabolism affecting all tissues, often resulting in morbidity and possibly mortality. The American Diabetes Association reported in 2013 that nearly 10% of the US population has diabetes, including 25% of seniors, which makes this metabolic disease the most common. Persistent hyperglycemia in diabetic patients inhibits osteoblastic activity and alters the parathyroid hormone response regulating calcium and phosphorus, decreases collagen formation during the initial callus formation, and induces osteoclastic activity due to a chronic inflammatory response.12 Type 1 diabetes is caused by an autoimmune reaction resulting in destruction of the beta cells of the pancreas leading to underproduction of insulin. Type 2 diabetics (most commonly seen in the adult population seeking dental implants) are characterized by resistance to insulin and the inability to manufacture compensatory endogenous insulin. Glycated hemoglobin (HbA1c – blood glucose levels measured over 8–12 weeks) greater than 6.5% has been considered a reliable metric indicating the diabetic patient is poorly controlled (Table 2.2, Table 2.3).
Table 2.2 Glycated hemoglobin (HbA1c) targets.
| HbA1c targets | mmol/mol | % |
| Nondiabetics | 20–41 | 4–5.9 |
| Diabetics | 48 | 6.5 |
| Diabetics at higher risk of hyperglycemia | 59 | 7.5 |
Table 2.3 Conversion of glycated haemoglobin (HbA1c) to blood glucose level.
| HbA1c (%) | Average blood glucose (mg/dL) |
| 6 | 120 |
| 7 | 150 |
| 8 | 180 |
Oates et al.13 have reported alterations in early (2–6 weeks) implant stability in direct relation to hyperglycemic conditions, which have been corroborated in a systematic review.14 However, the same authors later reported on a randomized controlled trial on implant overdenture therapy in normal, controlled, and uncontrolled diabetic patients with no correlation to implant survival after 1 year.15 This may point to the complexities of diabetes mellitus as well as the import of possible comorbidities. Careful evaluation of the patient’s medical status/history and consultation with a physician will lead to a personalized medical approach. At this time, it is not advised to place implants in a patient with uncontrolled diabetes until longer‐range follow‐up studies are conducted. It is prudent to consider the following regimen for patients with hyperglycemia: 1) good glycemic control pre‐ and postoperatively;16 2) at least 4 months of healing time;17 3) use of prophylactic antibiotics and 0.12% chlorhexidine;18 and 4) increased length and width of implants when native bone volume permits.3 When patients have had a history of controlled diabetes for more than 10 years, they may also benefit from the aforementioned recommendations.3
Intravenous bisphosphonates
Epidemiological studies have suggested a compelling, if not circumstantial, association between intravenous (IV) nitrogen‐containing bisphosphonates (BPs) and bisphosphonate‐related osteonecrosis of the jaw (BRONJ) (Figure 2.1).19 BRONJ can be a confirmed diagnosis if the patient is on a current or previous treatment with a bisphosphonate, presenting with exposed bone in the maxillofacial region that has persisted for 8 weeks, and no history of radiotherapy to the jaws. BPs are nonmetabolized analogs of pyrophosphate with a half‐life in the bone of 11 years and are described as effective inhibitors of osteoclastic function.20 As BP potency increases, the risk of BRONJ increases. For example, zoledronate is more than 100 times more potent than alendronate (Table 2.4).22 Frequency estimates for BRONJ risk in patients exposed to IV BPs have ranged from 0.7% to 12%, as opposed to risk with oral BPs ranging from 0.04% (without extractions) to 0.3% (with extractions). The preponderance of cases of BRONJ has been reported in the mandible.
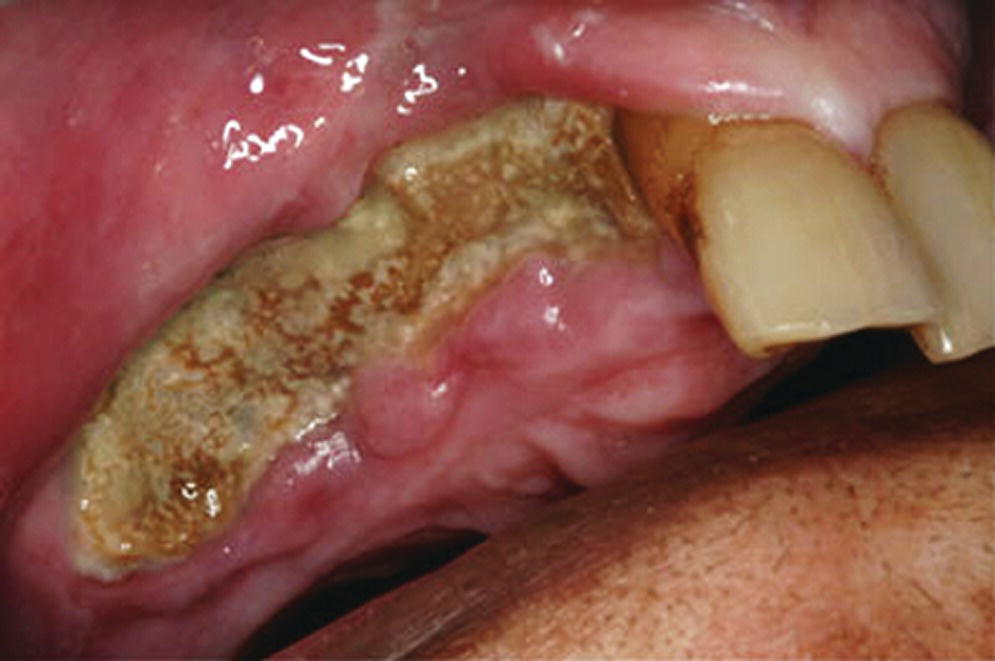
Figure 2.1 Osteonecrosis of the jaw.
Table 2.4 Relative potency of nitrogen‐containing bisphosphonates.21
| Drug name | Generic name | Relative potency* |
| Fosamax (oral) | Alendronate | 1000 |
| Actonel (oral) | Risedronate | 5000 |
| Boniva (oral/IV) | Ibandronate | 10 000 |
| Aredia (IV) | Pamidronate | 100 |
| Zometa (IV) | Zoledronic acid | 100 000 |
* Relative to etidronate (non‐nitrogen containing bisphosphonate with relative potency of 1).
IV BPs are used for the management of hypercalcemia and the treatment of symptomatic bony lesions or prevention of pathologic fracture from multiple myeloma, or metastatic tumors from breast cancer, lung cancer, prostate cancer, and other solid tumors.23 Hypercalcemia is a common complication of advanced malignancies, impacting 10–20% of patients during their disease state. IV BPs are dosed frequently (e.g. 4 mg of zoledronate monthly) as opposed to the management of osteoporosis which would use zoledronate in a 5 mg annual dose. While current studies suggest no increased risk for patients being managed with IV BPs for osteoporosis,24,25 the investigations are recent, and the cumulative exposure to IV BPs has been limited. This will further be addressed under the heading of Osteoporosis.
Based on a recent systematic review, implants can be placed successfully in patients with a history of IV BPs, but the studies are of moderate to weak strength of evidence with inherent bias and limitations, and hence results must be interpreted with caution.26 Until well‐controlled trials with higher strength of evidence on larger populations (with homogeneity in terms of BP dosage, frequency, and duration) are conducted, the use of IV BPs should be considered an absolute contraindication to implant placement.
Bleeding disorders
There is no reliable evidence that bleeding disorders should be considered in a class of absolute contraindications for implant placement. Even patients with congenital bleeding abnormalities (hemophiliacs) may be treated successfully with dental implants.27 However, if there is a history of bleeding problems in the patient/familial line or if medications are linked to clotting problems, laboratory tests, such as platelet count, bleeding time, prothrombin time (PT), and partial thromboplastin time (PTT), are recommended. The platelet count is ascertained in the complete blood count (CBC) and normal values are 200 000–300 000/mL. Values one tenth of normal represent a spontaneous bleeding risk. Bleeding time assesses both platelet function and capillary activity. Extrinsic (outside vessels) and intrinsic pathways (factors VII through XII) of coagulation are evaluated with the PT and PTT, respectively.
Anemia is the most common hematological disorder, resulting in a decreased production of erythrocytes or increased rate of their destruction, possibly from a deficiency in iron. Bone maturation can be impaired in the chronic anemic patient. Decreased density of bone has been found to influence time of integration. Hematocrit levels (volume of blood made up of erythrocytes) should be no lower than 40%. A hemoglobin test (assessment of oxygen‐containing protein in blood) is also needed to proceed with surgery (minimum 10 mg/dL).
There is a group of medications that inhibits the production of prothrombin. Patients on anticoagulants (e.g. warfarin) may proceed with implant placement without modifying their prescribed regimen, provided that the International Normalized Ratio (INR) is less than or equal to 2.5 (2.5 times normal to clot) and the surgery does not involve autogenous bone grafts or extensive flaps.28 If heparin has been prescribed for anticoagulation, a patient should have a PTT scheduled the day of surgery and if it is 1.5 times the normal value, surgery should be postponed. Long‐term antibiotic coverage interferes with intestinal bacteria necessary to produce vitamin K, affecting the prothrombin levels in the liver and a PT should be evaluated. Aspirin inhibits platelet function because it interferes with the hepatic production of prothrombin by blocking vitamin K. If four or more tablets (325 mg) are prescribed for more than 1 week, bleeding time and PTT can be affected, signaling a possibility of a bleeding complication. In rare cases, implant placement can cause arterial impingement (e.g. perforation of the lingual plate, Figure 2.2) and in patients with bleeding disorders, hemorrhage may be prolonged, raising the risk of a serious complication.28 However, with patients on antiplatelet therapy (aspirin, nonsteroidal anti‐inflammatory drugs) the risks of thromboembolism may outweigh the risk of bleeding after invasive dental procedures and therefore their medication usually is not altered.29
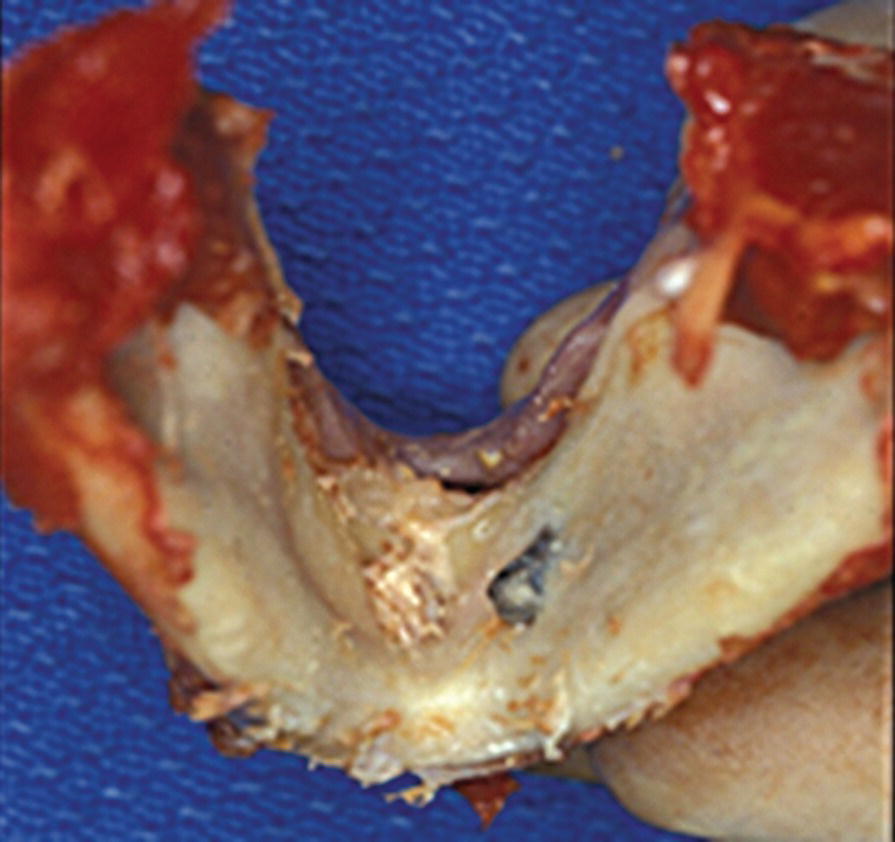
Figure 2.2 Implant perforation of lingual plate.
Despite the lack of evidence to suggest that bleeding disorders are an absolute contraindication to implant placement, medical advice should obtained before surgery, especially for patients with congenital bleeding maladies. With this cohort of patients, as with others that are medically compromised, a premium is placed on surgical expertise.
Recent myocardial infarction/stroke
It has been purported that some cardiovascular events, such as myocardial infarction, stroke, and cardiovascular surgery, may be classified in the category of absolute contraindications.30 A number of retrospective studies, however, have failed to link coronary artery disease or hypertension to a significant increase in either early or late implant failures.31–34 Despite the weight of this evidence, it is propitious to obtain medical advice before any surgical procedure is planned for these patients, as the sequela of bleeding or an ischemic event during placement of implants is possible. To this point, approximately 20% of patients with a recent history of MI will have complications of a recurrent episode with a mortality rate as high as 70%. The risk of recurrence with a surgical procedure decreases with time from 30%, if within 3 months of the MI, to 5% after 1 year post‐MI.35 It is of note when treating patients with unstable hemodynamics, that the efficacy of intravenous sedation using midazolam and propofol synergistically during implant surgery has been well demonstrated in a comparative study.36
Active treatment of malignancy
Due to the variability of disease conditions and combinations of treatment regimens, the oncological patient is difficult to categorize regarding implant placement risk. Radiotherapy is commonly used to treat head and neck tumors and has been found to significantly impact dental implant outcomes during the healing period as well as predisposing to osteonecrosis of the jaw because of the induction of endarteritis obliterans.37 For example, an 8‐year follow‐up demonstrated 75% implant survival rate of implants in the irradiated mandible.38 However, implant success rates in irradiated patients have been reported to be linked to the source, dose, and fractionation of irradiation, concomitant therapies, jaw site, and timing of the adjuvant therapies.39,40 The following guidelines have been proposed to improve implant therapy outcome:2,41,42
- the source should avoid implant portals, if possible;
- when the dose is less than 66 Gy, this reduces the risk of osteonecrosis of the jaw (more common in the mandible), and when less than 50 Gy reduces implant failure;
- daily fractions of 1/25 the total target irradiated dose is recommended;
- while controversy persists regarding the concomitant use of hyperbaric oxygen,40 hyperbaric treatment has been recommended when the therapeutic dose has been greater than 50 Gy.
When implants are inserted after radiotherapy, the implant failure rate can be expected to be lower on the mandible than maxilla (4.4% vs. 17.5%).42 Implant surgery is optimally carried out either more than 21 days before irradiation therapy or 9 months after. Implant placement is not indicated during radiotherapy or in the presence of mucositis. Immediate loading is not recommended on post‐radiation patients.
In regard to implant therapy following chemotherapy, two studies compared data to healthy controls and found no significant difference in implant failure rates when radiotherapy did not accompany treatment.34,44 On the other hand, a history of therapeutic radiation is considered a relative contraindication to implant treatment, especially on the maxilla. In summary, active radiotherapy is an absolute contraindication to implant placement, but implant placement is not contraindicated in the patient after completing only chemotherapy,44 although comorbidities must be analyzed carefully. Consultation with the oncologist is recommended in all cases when surgical and prosthodontic treatment is planned for the cancer patient. Dental implants are not necessarily contraindicated in patients with terminal illness as their quality of life may be immensely improved during their time remaining.
Alcohol/drug abuse
While animal studies45 have found a negative impact of alcohol intake on bone density and osseointegration (bone‐to‐implant contact), human studies have only established an increased risk of complications such as peri‐implantitis in this subset of patients.46,47 Attendant to more occult conditions, alcoholism has also been shown to be implicated in a myriad of problems from bleeding disorders due to a decrease in platelet production, to impairing nutrition (e.g. vitamin B) and the immune response (elevated levels of cortisol and interleukin [IL]‐10).2 Chronic alcoholism increases infection risk postoperatively due to the T‐helper cell ratio of T1 to T2 being depressed.48 Preoperative alcohol cessation prior to implant therapy was evaluated with two randomized controlled studies using pharmacological strategies for alcohol withdrawal and relapse.49 The findings demonstrate reduction of postoperative complication rate, including decreased frequency of delirium tremors and postoperative seizures while reducing the need for potent sedatives. Timing, duration, and intensity of intervention, however, need further study. In summary, alcoholism (10% of US population is dependent50) is often linked to comorbidities which can magnify the incidence of untoward postsurgical consequences.
In 2010, it was estimated that approximately 8.9% of persons older than 12 years old were illicit drug users, which has increased from 6.3% in 2000.51,52
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


