Dental Caries
Etiology, Clinical Characteristics, Risk Assessment, and Management
What is Dental Caries?
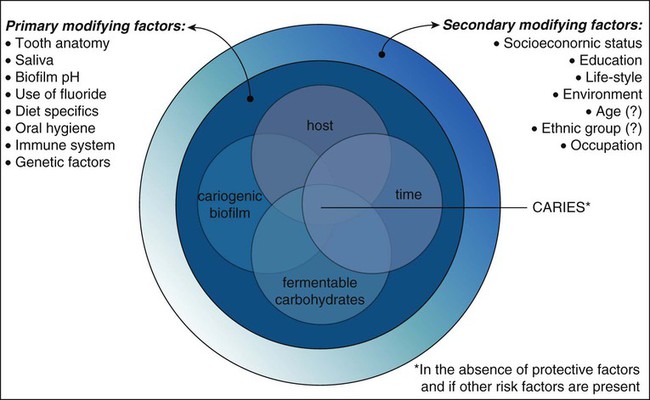
Dental caries is a multifactorial, transmissible, infectious oral disease caused primarily by the complex interaction of cariogenic oral flora (biofilm) with fermentable dietary carbohydrates on the tooth surface over time. Traditionally, this tooth-biofilm-carbohydrate interaction has been illustrated by the classical Keyes-Jordan diagram (Fig. 2-1).1 However, dental caries onset and activity are, in fact, much more complex than this three-way interaction, as not all persons with teeth, biofilm, and consuming carbohydrates will have caries over time. Several modifying risk and protective factors influence the dental caries process, as will be discussed later in this chapter.
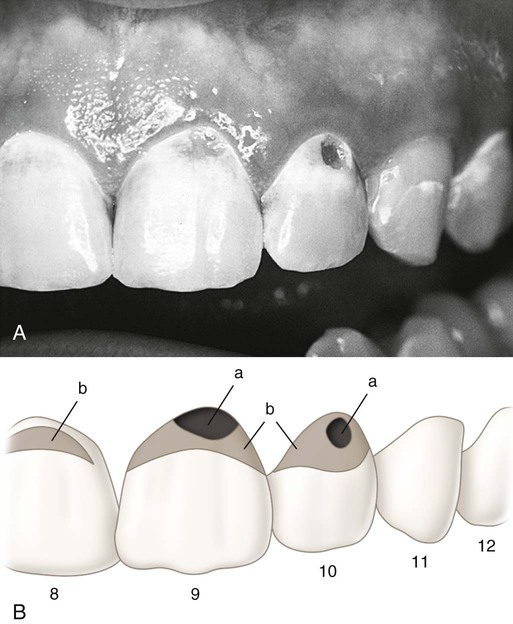
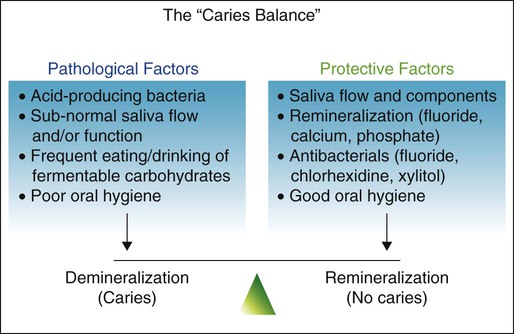
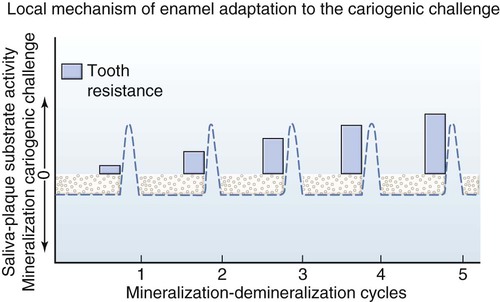
At the tooth level, caries activity is characterized by localized demineralization and loss of tooth structure (Figs. 2-2 and 2-3). Cariogenic bacteria in the biofilm metabolize refined carbohydrates for energy and produce organic acid by-products. These organic acids, if present in the biofilm ecosystem for extended periods, can lower the pH in the biofilm to below a critical level (5.5 for enamel, 6.2 for dentin). The low pH drives calcium and phosphate from the tooth to the biofilm in an attempt to reach equilibrium, hence resulting in a net loss of minerals by the tooth, or demineralization. When the pH in the biofilm returns to neutral and the concentration of soluble calcium and phosphate is supersaturated relative to that in the tooth, mineral can then be added back to partially demineralized enamel, in a process called remineralization. At the tooth surface and sub-surface level, therefore, dental caries results from a dynamic process of attack (demineralization) and restitution (remineralization) of the tooth matter. These events take place several times a day over the life of the tooth and are modulated by many factors, including number and type of microbial flora in the biofilm, diet, oral hygiene, genetics, dental anatomy, use of fluorides and other chemotherapeutic agents, salivary flow and buffering capacity; and inherent resistance of the tooth structure and composition that will differ from person to person, tooth to tooth, and site to site. The balance between demineralization and remineralization has been illustrated in terms of pathologic factors (i.e., those favoring demineralization) and protective factors (i.e., those favoring remineralization) (Fig. 2-4).2 Individuals in whom the balance tilts predominantly toward protective factors (remineralization) are much less likely to develop dental caries than those in which the balance is tilted toward pathologic factors (demineralization). Understanding the balance between demineralization and remineralization is key to caries management.
Repeated demineralization events may result from a predominantly pathologic environment causing the localized dissolution and destruction of the calcified dental tissues, evidenced as a caries lesion or a “cavity.” Severe demineralization of enamel results in the formation of a cavitation in the enamel surface. Severe demineralization of dentin results in the exposure of the protein matrix, which is denatured initially by host matrix metalloproteinases (MMPs) and is subsequently degraded by MMPs and other bacterial proteases. Demineralization of the inorganic phase and denaturation and degradation of the organic phase result in dentin cavitation.3
It is essential to understand that caries lesions, or cavitations in teeth, are signs of an underlying condition, an imbalance between protective and pathologic factors favoring the latter. In clinical practice, it is very easy to lose sight of this fact and focus entirely on the restorative treatment of caries lesions, failing to treat the underlying cause of the disease (Table 2-1). Although symptomatic treatment is important, failure to identify and treat the underlying causative factors allows the disease to continue. This chapter emphasizes the components of a caries management program that is based first on risk assessment and then on modifying the biofilm ecology to enhance protective factors and minimize pathologic factors.4
Table 2-1
Caries Management Based on the Medical Model
| Primary Etiology | Cariogenic Biofilm (Infection) |
| Symptoms | Demineralization lesions in teeth |
| Treatment, symptomatic | Restoration of cavitated lesions |
| Treatment, therapeutic | Improvement of host resistance by (1) biofilm control, (2) elevating biofilm pH, and (3) enhancing remineralization |
| Post-treatment assessment, symptomatic | Examination of teeth for new lesions |
| Post-treatment assessment, therapeutic | Re-evaluation of etiologic conditions and primary and secondary risk factors; and continuous management based on findings |
This chapter also presents information on clinical characteristics of caries lesion as they relate to clinical operative dentistry. Use of correct and consistent terms when referring to caries lesions is important. Box 2-1 summarizes the most common terms used in this textbook to define caries lesions based on their location, cavitation status, and activity status.
Ecologic Basis of Dental Caries: The Role of the Biofilm
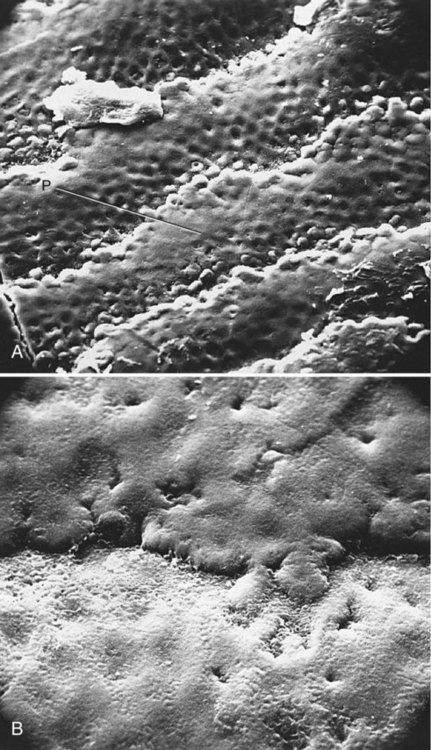
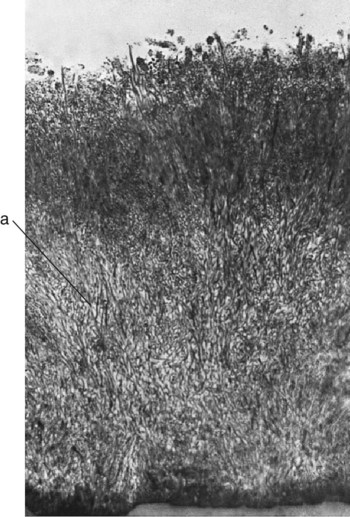
Dental plaque is a term historically used to describe the soft, tenacious film accumulating on the surface of teeth. Dental plaque has been more recently referred to as a plaque biofilm, or simply biofilm, which is a more complete and accurate description of its composition (bio) and structure (film).5 Biofilm is composed mostly of bacteria, their by-products, extracellular matrix, and water (Figs. 2-5 to 2-9). Biofilm is not adherent food debris, as is widely and erroneously thought, nor does it result from the haphazard collection of opportunistic microorganisms. The accumulation of biofilm on teeth is a highly organized and ordered sequence of events. Many of the organisms found in the mouth are not found elsewhere in nature. Survival of microorganisms in the oral environment depends on their ability to adhere to a surface. Free-floating organisms are cleared rapidly from the mouth by salivary flow and frequent swallowing. Only a few specialized organisms, primarily streptococci, are able to adhere to oral surfaces such as the mucosa and tooth structure.
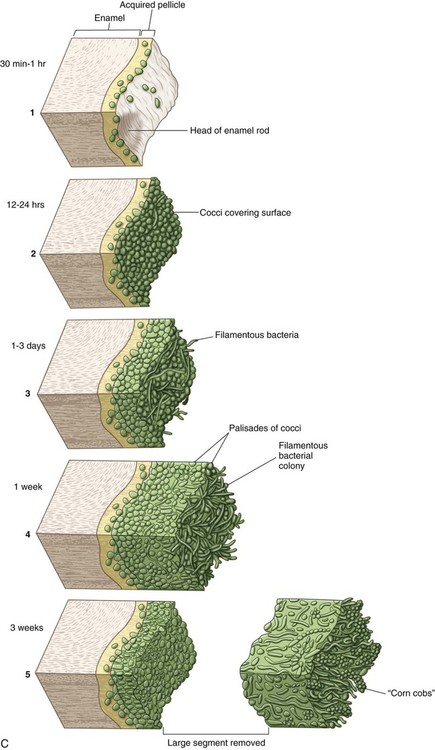
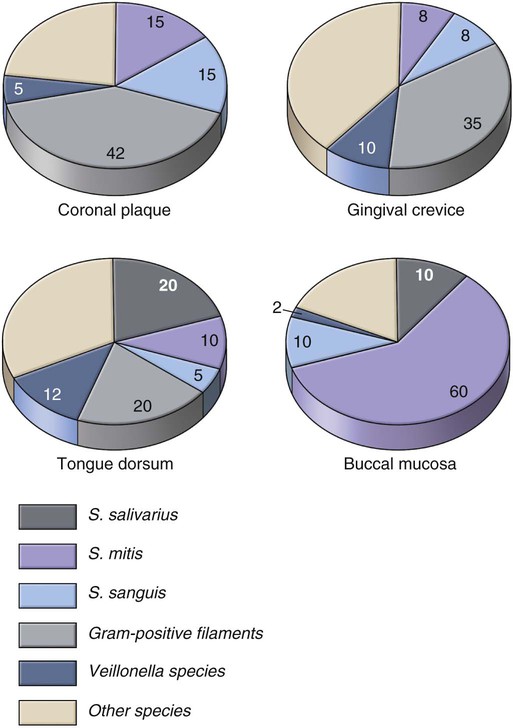
Significant differences exist in the biofilm communities found in various habitats (ecologic environments) within the oral cavity (Fig. 2-10). Teeth normally have a biofilm community dominated by Streptococcus sanguis and S. mitis. The population size of mutans streptococci (MS) on teeth varies. Normally, it is a small percentage of the total biofilm population, but it can be one-half the facultative streptococcal flora in other biofilms. Mature plaque biofilm communities have tremendous metabolic potential and are capable of rapid anaerobic metabolism of any available carbohydrates (Fig. 2-11).
Many distinct habitats may be identified on individual teeth, with each habitat containing a unique biofilm community (Table 2-2). Although the pits and fissures on the crown may harbor a relatively simple population of streptococci, the root surface in the gingival sulcus may harbor a complex community dominated by filamentous and spiral bacteria. Facial and lingual smooth surfaces and proximal surfaces also may harbor vastly different biofilm communities. The mesial surface of a molar may be carious and have a biofilm dominated by large populations of MS and lactobacilli, whereas the distal surface may lack these organisms and be caries-free. Generalization about biofilm communities is difficult. Nevertheless, the general activity of biofilm growth and maturation is predictable and sufficiently well known to be of therapeutic importance in the prevention of caries.
Table 2-2
| Habitat | Predominant Species | Environmental Conditions within Plaque |
| Mucosa | S. mitis | Aerobic |
| S. sanguis | pH approximately 7 | |
| S. salivarius | Oxidation-reduction potential positive | |
| Tongue | S. salivarius | Aerobic |
| S. mutans | pH approximately 7 | |
| S. sanguis | Oxidation-reduction potential positive | |
| Teeth (non-carious) | S. sanguis | Aerobic |
| pH 5.5 | ||
| Oxidation-reduction negative | ||
| Gingival crevice | Fusobacterium | Anaerobic |
| Spirochaeta | pH variable | |
| Actinomyces | Oxidation-reduction very negative | |
| Veillonella | ||
| Enamel caries | S. mutans | Anaerobic |
| pH <5.5 | ||
| Oxidation-reduction negative | ||
| Dentin caries | S. mutans | Anaerobic |
| Lactobacillus | pH <5.5 | |
| Oxidation-reduction negative | ||
| Root caries | Actinomyces | Anaerobic |
| pH <5.5 | ||
| Oxidation-reduction negative |
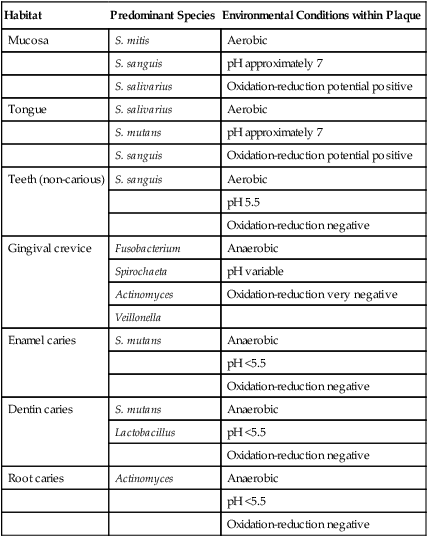
*The micro-environmental conditions in the habitats associated with host health are generally aerobic, near neutrality in pH, and positive in oxidation-reduction potential. Significant micro-environmental changes are associated with caries and periodontal disease. The changes are the result of the plaque community metabolism.
Professional tooth cleaning is intended to control biofilm (plaque) and prevent disease. After professional removal of all organic material and bacteria from the tooth surface, a new coating of organic material begins to accumulate immediately. Within 2 hours, a cell-free, structureless organic film, the acquired enamel pellicle (AEP, see Figs. 2-5, A and C), can cover the previously denuded area completely. The pellicle is formed primarily from the selective precipitation of various components of saliva. The functions of the pellicle are believed to be as follows: (1) to protect the enamel, (2) to reduce friction between teeth, and (3) possibly to provide a matrix for remineralization.6
Tooth Habitats for Cariogenic Biofilm
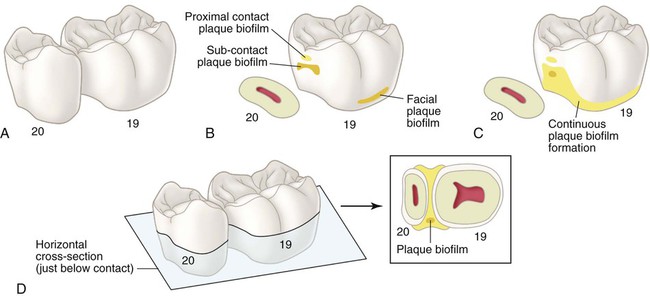
The tooth surface is unique because it is not protected by the surface shedding mechanisms (continual replacement of epithelial cells) used throughout the remainder of the alimentary canal. The tooth surface is stable and covered with the pellicle of precipitated salivary glycoproteins, enzymes, and immunoglobulins. It is the ideal surface for the attachment of many oral streptococci. If left undisturbed, biofilm rapidly builds up to sufficient depth to produce an anaerobic environment adjacent to the tooth surface. Tooth habitats favorable for harboring pathogenic biofilm include (1) pits and fissures (Fig. 2-12); (2) the smooth enamel surfaces immediately gingival to the proximal contacts and in the gingival third of the facial and lingual surfaces of the clinical crown (Fig. 2-13); (3) root surfaces, particularly near the cervical line; and (4) subgingival areas (Fig. 2-14). These sites correspond to the locations where caries lesions are most frequently found.
Pits and Fissures
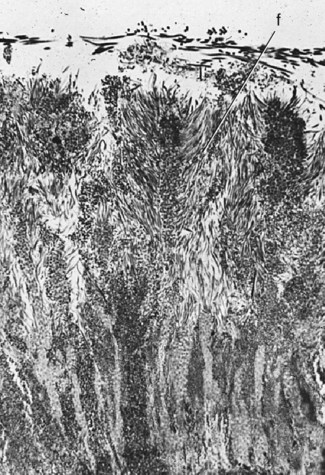
Pits and fissures are particularly susceptible surfaces for caries initiation (see Fig. 2-12; Figs. 2-15 to 2-19; see also Fig. 2-12). The pits and fissures provide excellent mechanical shelter for organisms and harbor a community dominated by S. sanguis and other streptococci.7 The relative proportion of MS most probably determines the cariogenic potential of the pit-and-fissure community. The appearance of MS in pits and fissures is usually followed by caries 6 to 24 months later. In susceptible patients, sealing the pits and fissures just after tooth eruption may be the most important event in their resistance to caries.
Smooth Enamel Surfaces
The proximal enamel surfaces immediately gingival to the contact area are the second most susceptible areas to caries (Figs. 2-20 and 2-21; see also Figs. 2-14 and 2-18). These areas are protected physically and are relatively free from the effects of mastication, tongue movement, and salivary flow. The types and numbers of organisms composing the proximal surface biofilm community vary. Important ecologic determinants for the biofilm community on the proximal surfaces are the topography of the tooth surface, the size and shape of the gingival papillae, and the oral hygiene of the patient. A rough surface (caused by caries, a poor-quality restoration, or a structural defect) restricts adequate biofilm removal. This situation favors the occurrence of caries or periodontal disease at the site.
Saliva: Nature’s Anticaries Agent
Saliva is an extremely important substance for the proper digestion of foods, and it also plays a key role as a natural anticaries agent (Table 2-3). Many medications are capable of reducing salivary flow and increasing caries risk (Table 2-4). The importance of saliva in the maintenance of the oral health is illustrated dramatically by observing changes in oral health after therapeutic radiation to the head and neck. After radiation, salivary glands become fibrotic and produce little or no saliva, leaving the patient with an extremely dry mouth, a condition termed xerostomia (xero, dry; stoma, mouth). Such patients may experience near-total destruction of the teeth in just a few months after radiation treatment.8,9 Salivary protective mechanisms that maintain the normal oral flora and tooth surface integrity include bacterial clearance, direct antibacterial activity, buffers, and remineralization.10
Table 2-3
Elements of Saliva that Control Plaque Biofilm Communities
| Names | Action | Effects on Plaque Biofilm Community |
| SALIVARY ENZYMES | ||
| Amylase | Cleaves—1,4 glucoside bonds | Increases availability of oligosaccharides |
| Lactoperoxidase | Catalyzes hydrogen peroxide–mediated oxidation; adsorbs to hydroxyapatite in active form | Lethal to many organisms: suppresses plaque formation on tooth surfaces |
| Lysozyme | Lyses cells by degradation of cell walls, releasing peptidoglycans; binds to hydroxyapatite in active conformation | Lethal to many organisms; peptidoglycans activate complement; suppresses plaque formation on tooth surfaces |
| Lipases | Hydrolysis of triglycerides to free fatty acids and partial glycerides | Free fatty acids inhibit attachment and growth of some organisms |
| NON-ENZYME PROTEINS | ||
| Lactoferrin | Ties up free iron | Inhibits growth of some iron-dependent microbes |
| Secretory immunoglobulin A(IgA) (smaller amounts of IgM, IgG) | Agglutination of bacteria inhibits bacterial enzymes | Reduces numbers in saliva by precipitation; slows bacterial growth |
| Glycoproteins (mucins) | Agglutination of bacteria | Reduces numbers in saliva by precipitation |
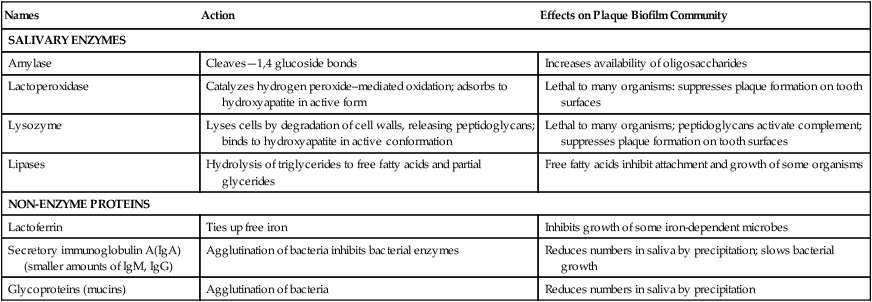
Table 2-4
Medications with Potential to Cause Hyposalivation or Dry Mouth (Xerostomia)
| Action/Medication Group | Medicaments |
| SYMPATHOMIMETIC | |
| Antidepressants | |
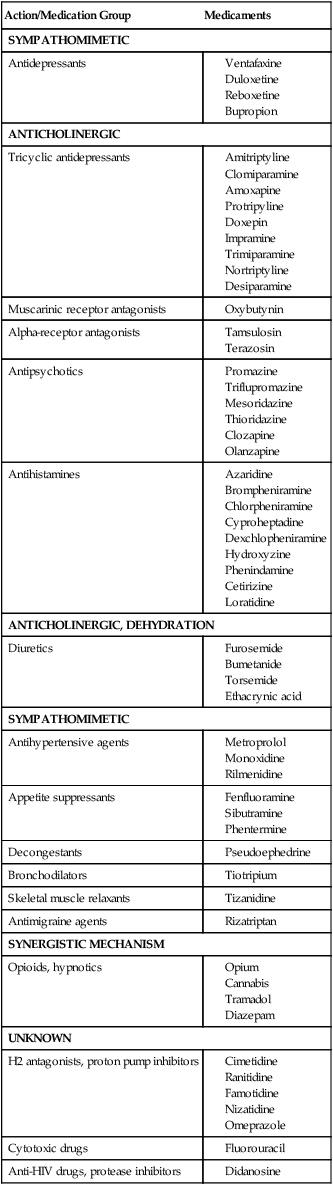
(Adapted from the Kois Center, Support Materials, Always Pages, http://koiscenter.com/store/supmatlist.aspx, accessed January 13, 2012.)
Direct Antibacterial Activity
Salivary glands produce an impressive array of antimicrobial products (see Table 2-3). Lysozyme, lactoperoxidase, lactoferrin, and agglutinins possess antibacterial activity. These salivary proteins are not part of the immune system but are part of an overall protection scheme for mucous membranes that occurs in addition to immunologic control. These protective proteins are present continuously at relatively uniform levels, have a broad spectrum of activity, and do not possess the “memory” of immunologic mechanisms. The normal resident oral flora apparently has developed resistance to most of these antibacterial mechanisms.
Although the antibacterial proteins in saliva play an important role in the protection of soft tissue in the oral cavity from infection by pathogens, they have little effect on caries because similar levels of antibacterial proteins can be found in caries-active and caries-free individuals.11,12 It is suggested that caries susceptibility in healthy individuals is not related to saliva composition. Individuals with decreased salivary production (owing to illness, medication, or irradiation) may have significantly higher caries susceptibility (see Table 2-4).
Buffer Capacity
The volume and buffering capacity of saliva available to tooth surfaces have major roles in caries protection.13 The buffering capacity of saliva is determined primarily by the concentration of bicarbonate ion. Buffering capacity can be estimated by titration techniques and may be a useful method for assessment of saliva in caries-active patients. The benefit of the buffering is to reduce the potential for acid formation.
Remineralization
Saliva and biofilm fluid are supersaturated with calcium and phosphate ions. Without a means to control precipitation of these ions, the teeth literally would become encrusted with mineral deposits. Saliva contains statherin, a proline-rich peptide that stabilizes calcium and phosphate ions and prevents excessive deposition of these ions on teeth.14 This supersaturated state of the saliva provides a constant opportunity for remineralizing enamel and can help protect teeth in times of cariogenic challenges.
Clinical Characteristics of the Caries Lesion
Clinical Sites for Caries Initiation
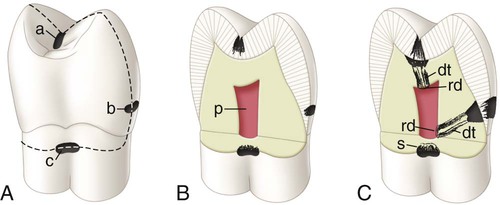
The characteristics of a caries lesion vary with the nature of the surface on which the lesion develops. There are three distinctly different clinical sites for caries initiation: (1) developmental pits and fissures of enamel, which are the most susceptible sites; (2) smooth enamel surfaces that shelter cariogenic biofilm; and (3) the root surface (see Fig. 2-14). Each of these areas has distinct surface topography and environmental conditions. Consequently, each area has a distinct biofilm population. The diagnosis, treatment, and prevention of these different lesion types should take into account the different etiologic factors operating at each site.
Pits and Fissures
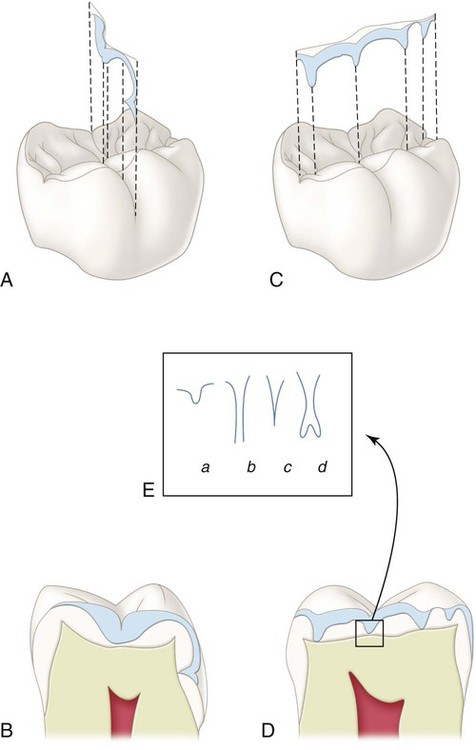
The shape of the pits and fissures contributes to their high susceptibility to caries. The long, narrow fissure prevents adequate biofilm removal (see Fig. 2-12). Considerable morphologic variation exists in these structures. Some pits and fissures end blindly, others open near the dentin, and others penetrate entirely through the enamel.
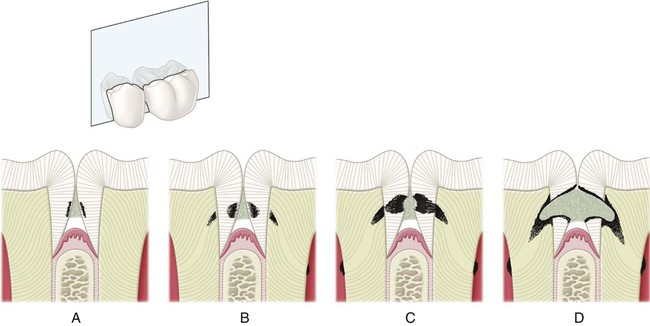
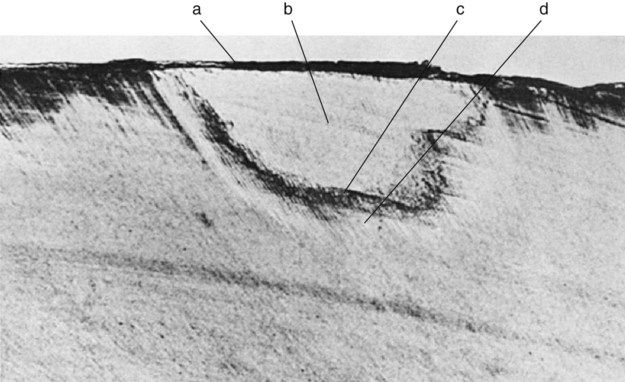
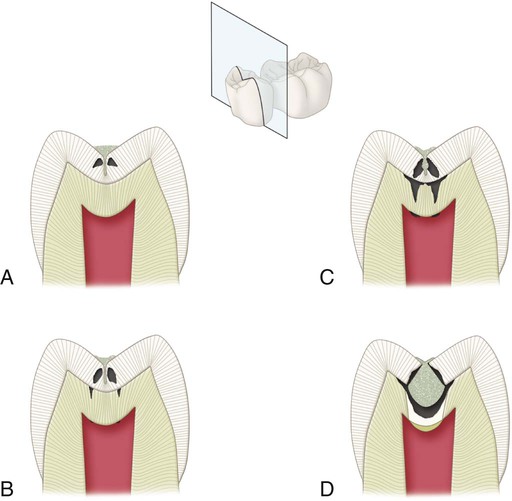
Pit-and-fissure caries expands as it penetrates into the enamel. The entry site may appear much smaller than the actual lesion, making clinical diagnosis difficult. Caries lesions of pits and fissures develop from attack on their walls (see Fig. 2-15, A through C). Progression of the dissolution of the walls of a pit-and-fissure lesion is similar in principle to that of the smooth-surface lesion because a wide area of surface attack extends inward, paralleling the enamel rods. A lesion originating in a pit or fissure affects a greater area of the DEJ than does a comparable smooth-surface lesion. In cross-section, the gross appearance of a pit-and-fissure lesion is an inverted “V” with a narrow entrance and a progressively wider area of involvement closer to the DEJ (see Fig. 2-15, D).
Smooth Enamel Surfaces
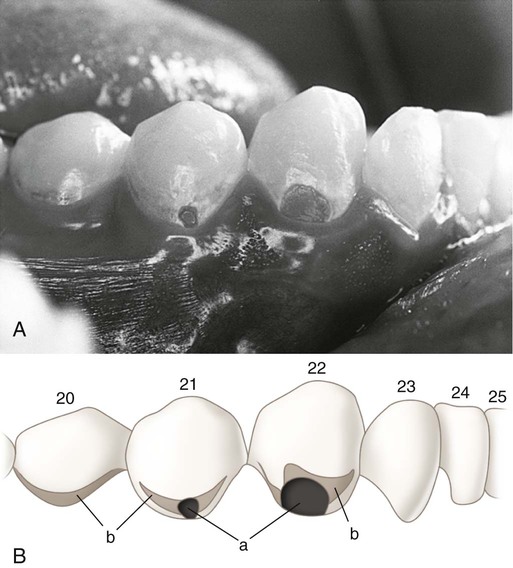
The smooth enamel surfaces of teeth present a less favorable site for cariogenic biofilm attachment. Cariogenic biofilm usually develops only on the smooth surfaces that are near the gingiva or are under proximal contacts. The proximal surfaces are particularly susceptible to caries because of the extra shelter provided to resident cariogenic biofilm owing to the proximal contact area immediately occlusal to it (Fig. 2-22). Lesions starting on smooth enamel surfaces have a broad area of origin and a conical, or pointed, extension toward the DEJ. The path of ingress of the lesion is roughly parallel to the long axis of the enamel rods in the region. A cross-section of the enamel portion of a smooth-surface lesion shows a V-shape, with a wide area of origin and the apex of the V directed toward the DEJ. After caries penetrates the DEJ, softening of dentin spreads rapidly laterally and pulpally (see Fig. 2-21).
Root Surfaces
The root surface is rougher than enamel and readily allows cariogenic biofilm formation in the absence of good oral hygiene. The cementum covering the root surface is extremely thin and provides little resistance to caries attack. In addition, the critical pH for dentin is higher than for enamel, so demineralization is likely to start even before the pH reaches the critical level for enamel (pH = 5.5). Root caries lesions have less well-defined margins, tend to be U-shaped in cross-section, and progress more rapidly because of the lack of protection from an enamel covering. In recent years, the prevalence of root caries has increased significantly because of the increasing number of older persons who retain more teeth, experience gingival recession, and usually have cariogenic biofilm on the exposed root surfaces.15–18
Progression of Caries Lesions
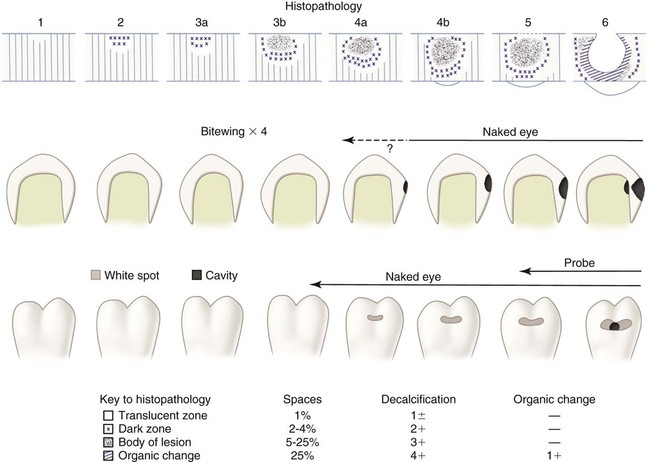
The progression and morphology of the caries lesion vary, depending on the site of origin and the conditions in the mouth (see Figs. 2-14, 2-15, and 2-21). The time for progression from non-cavitated caries to clinical caries (cavitation) on smooth surfaces is estimated to be 18 months ± 6 months.19 Peak rates for the incidence of new lesions occur 3 years after the eruption of the tooth. Occlusal pit-and-fissure lesions develop in less time than smooth-surface caries. Poor oral hygiene and frequent exposures to sucrose-containing or acidic food can produce noncavitated (“white spot”) lesions (first clinical evidence of demineralization) in 3 weeks. Radiation-induced xerostomia (dry mouth) can lead to clinical caries development in 3 months from the onset of the radiation. Caries development in healthy individuals is usually slow compared with the rate possible in compromised persons.
Enamel Caries
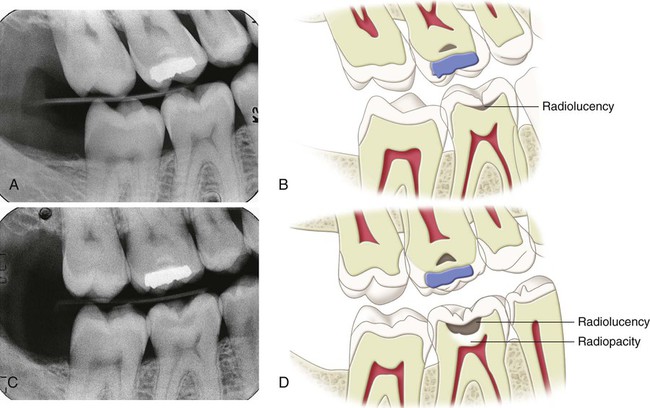
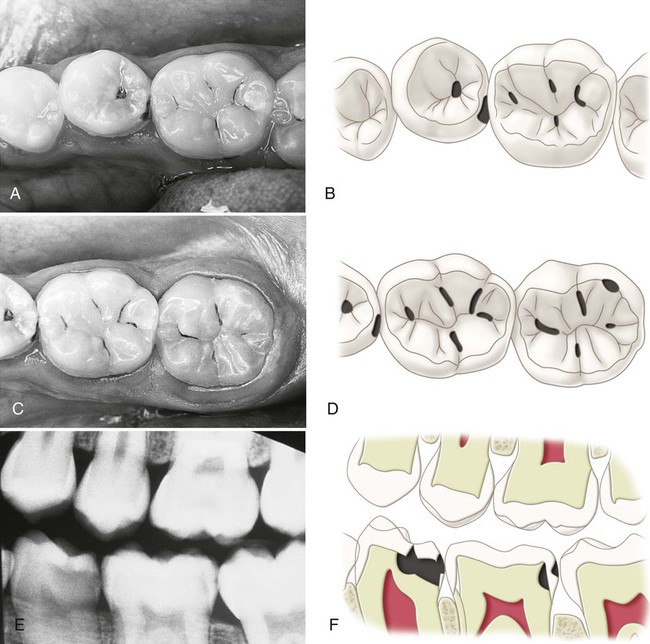
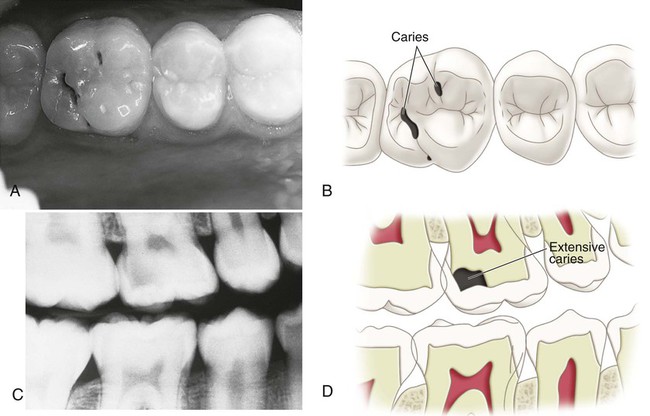
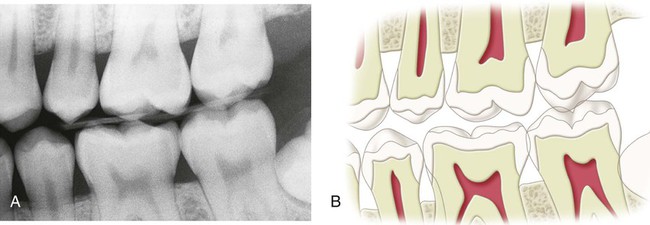
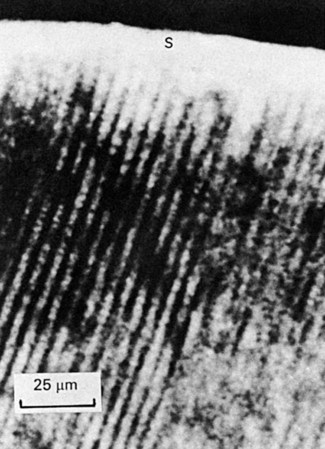
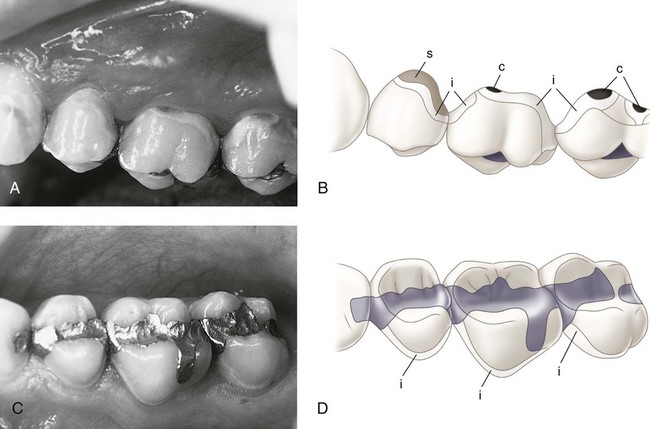
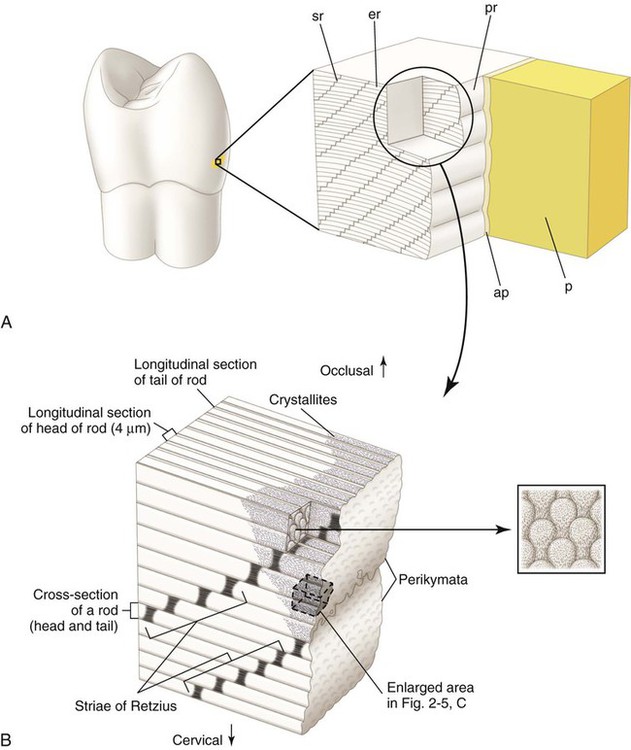
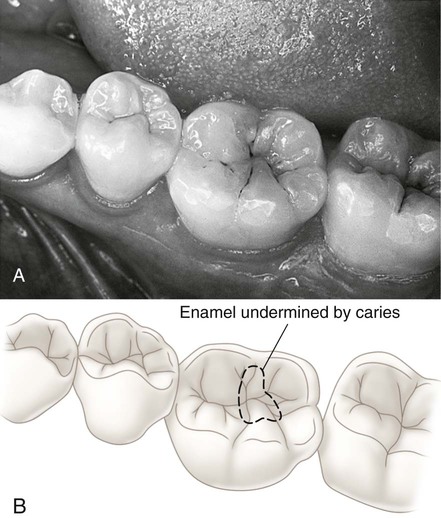
An understanding of the enamel composition and histology is helpful to understand enamel caries histopathology (see Chapter 1 (Figs. 2-23 to 2-25). On clean, dry teeth, the earliest evidence of caries on the smooth enamel surface of a crown is a white spot (Fig. 2-26; see also Figs. 2-2 and 2-3). These lesions usually are observed on the facial and lingual surfaces of teeth. White spots are chalky white, opaque areas that are revealed only when the tooth surface is desiccated and are termed noncavitated enamel caries lesions. These areas of enamel lose their translucency because of the extensive subsurface porosity caused by demineralization. Care must be exercised in distinguishing white spots of noncavitated caries from developmental white spot hypocalcifications of enamel. Noncavitated (white spot) caries partially or totally disappears visually when the enamel is hydrated (wet), whereas hypocalcified enamel is affected less by drying and wetting (Table 2-5). Hypocalcified enamel does not represent a clinical problem except for its esthetically objectionable appearance. The surface texture of a non-cavitated lesion is unaltered and is undetectable by tactile examination with an explorer. A more advanced lesion develops a rough surface that is softer than the unaffected, normal enamel. Softened chalky enamel that can be chipped away with an explorer is a sign of active caries. Injudicious use of an explorer tip can cause actual cavitation in a previously noncavitated area, requiring, in most cases, restorative intervention. Similar noncavitated lesions occur on the proximal smooth surfaces, but usually are undetectable by visual or judicious tactile (explorer) examination. Noncavitated enamel lesions sometimes can be seen on radiographs as a faint radiolucency that is limited to the superficial enamel. When a proximal lesion is clearly visible radiographically, the lesion may have advanced significantly, and histologic alteration of the underlying dentin probably already has occurred, whether the lesion is cavitated or not (Fig. 2-27).
Table 2-5
Clinical Characteristics of Normal and Altered Enamel
| Hydrated | Desiccated | Surface Texture | Surface Hardness | |
| Normal enamel | Translucent | Translucent | Smooth | Hard |
| Hypocalcified enamel | Opaque | Opaque | Smooth | Hard |
| Noncavitated caries | Translucent | Opaque | Smooth | Softened |
| Active caries | Opaque | Opaque | Cavitated | Very soft |
| Inactive caries | Opaque, dark | Opaque, dark | Roughened | Hard |
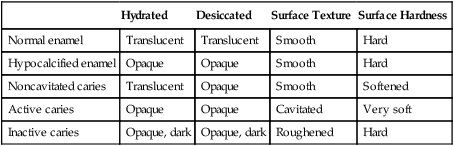

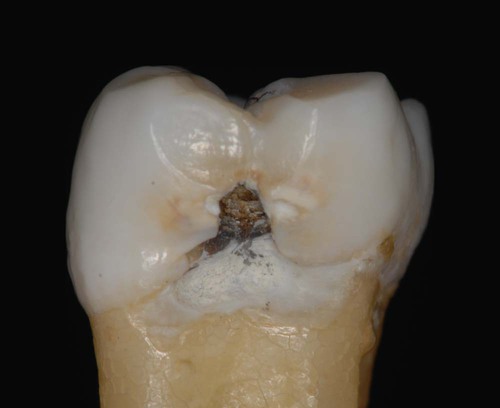
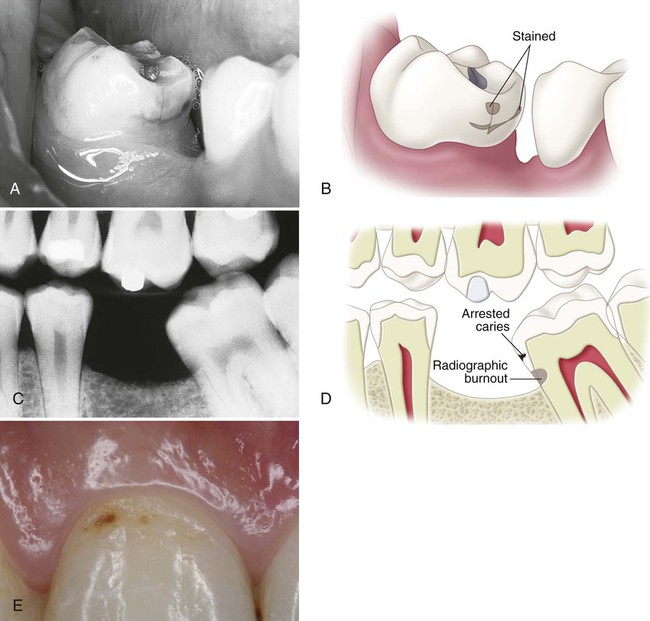
It has been shown experimentally and clinically that noncavitated caries of enamel can remineralize.20–21 Table 2-5 and Table 2-6 list the characteristics of enamel at various stages of demineralization. Noncavitated enamel lesions retain most of the original crystalline framework of the enamel rods, and the etched crystallites serve as nucleating agents for remineralization. Calcium and phosphate ions from saliva can penetrate the enamel surface and precipitate on the highly reactive crystalline surfaces in the enamel lesion. The supersaturation of saliva with calcium and phosphate ions serves as the driving force for the remineralization process. Artificial and natural caries lesions of human enamel have been shown to regress to earlier histologic stages after exposure to conditions that promote remineralization. The presence of trace amounts of fluoride ions during this remineralization process greatly enhances the precipitation of calcium and phosphate, resulting in the remineralized enamel becoming more resistant to subsequent caries attack because of the incorporation of more acid-resistant fluorapatite (Fig. 2-28). Remineralized (arrested) lesions can be observed clinically as intact, but discolored, usually brown or black, spots (Fig. 2-29). The change in color is presumably caused by trapped organic debris and metallic ions within the enamel. These discolored, remineralized, arrested caries areas are intact and are more resistant to subsequent caries attack than the adjacent unaffected enamel. They should not be restored unless they are esthetically objectionable.
Table 2-6
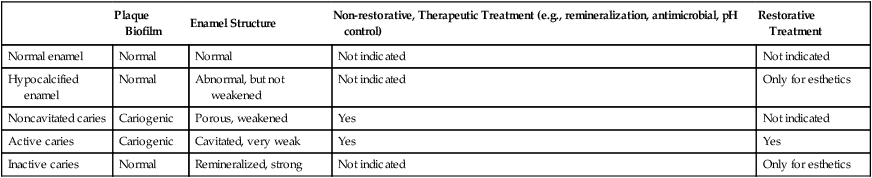
Clinical Significance of Enamel Lesions
| Plaque Biofilm | Enamel Structure | Non-restorative, Therapeutic Treatment (e.g., remineralization, antimicrobial, pH control) | Restorative Treatment | |
| Normal enamel | Normal | Normal | Not indicated | Not indicated |
| Hypocalcified enamel | Normal | Abnormal, but not weakened | Not indicated | Only for esthetics |
| Noncavitated caries | Cariogenic | Porous, weakened | Yes | Not indicated |
| Active caries | Cariogenic | Cavitated, very weak | Yes | Yes |
| Inactive caries | Normal | Remineralized, strong | Not indicated | Only for esthetics |
Dentin Caries
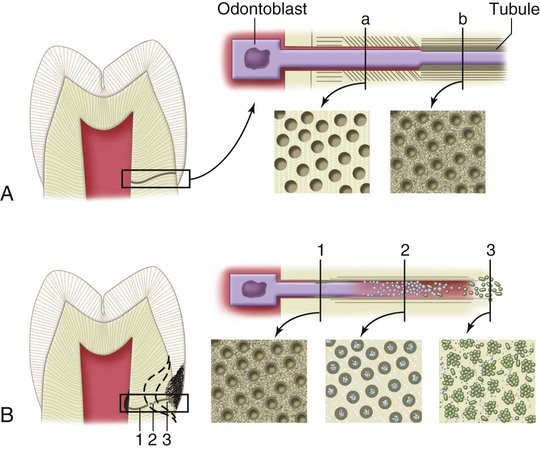
An understanding of the dentin composition and histology is helpful to understand the histopathology of dentin caries (see Chapter 1 (Fig. 2-30). Progression of caries in dentin is different from progression in the overlying enamel because of the structural differences of dentin (Figs. 2-31 to 2-33; see also Fig. 2-30). Dentin contains much less mineral and possesses microscopic tubules that provide a pathway for the ingress of bacteria and egress of minerals. The DEJ has the least resistance to caries attack and allows rapid lateral spreading when caries has penetrated the enamel (see Figs. 2-15 and 2-21). Because of these characteristics, dentinal caries is V-shaped in cross-section with a wide base at the DEJ and the apex directed pulpally. Caries advances more rapidly in dentin than in enamel because dentin provides much less resistance to acid attack owing to less mineralized content. Caries produces a variety of responses in dentin, including pain, sensitivity, demineralization, and remineralization.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses