Enamel and dentine bonding
Introduction
The development of an adhesive approach to restorative dentistry has brought many advantages, such as:
A wide variety of adhesive systems have been introduced in recent years, many of which have not survived the test of time. Such adhesives were unable to satisfy the stringent requirements that are placed on a dental adhesive.
The bonding systems that have survived the test of time include:
By comparison, the dentine-bonding agents have had a turbulent history. Many have come and gone, but at each stage of their development there has been an encouraging improvement. Perhaps some of the dentine-bonding agents now being marketed will survive the test of time.
Why some materials and techniques should have survived and others waned is due to the requirement that the adhesive needs to be able to bond to a variety of materials (e.g. composites, metals, ceramics) and to two very different substrates: namely, enamel and dentine.
The principles of adhesion have already been discussed in Chapter 1.9 and the adhesive aspects of GICs have already been dealt with in Chapter 2.3. Hence, in this chapter, the methods of bonding composites and resins to enamel and dentine with dentine-bonding agents only will be considered.
Enamel bonding
Structure of enamel
Enamel is the most densely calcified tissue of the human body, and is unique in the sense that it is formed extracellularly.
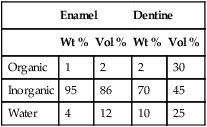
It is a heterogeneous structure, with mature human enamel consisting of 96% mineral, 1% organic material and 3% water by weight (Table 2.5.1). The mineral phase is made up of millions of tiny crystals of hydroxyapatite, Ca10 (PO4)6(OH)2, which are packed tightly together in the form of prisms, held together by an organic matrix. Due to ionic substitution (e.g. fluoride), the enamel apatite does not have the calcium-to-phosphate ratio of theoretically pure hydroxyapatite (1.6 : 1) and is usually in the ratio 2 : 1 by weight.
Table 2.5.1
Typical composition of enamel and dentine
| Enamel | Dentine | |||
| Wt % | Vol % | Wt % | Vol % | |
| Organic | 1 | 2 | 2 | 30 |
| Inorganic | 95 | 86 | 70 | 45 |
| Water | 4 | 12 | 10 | 25 |
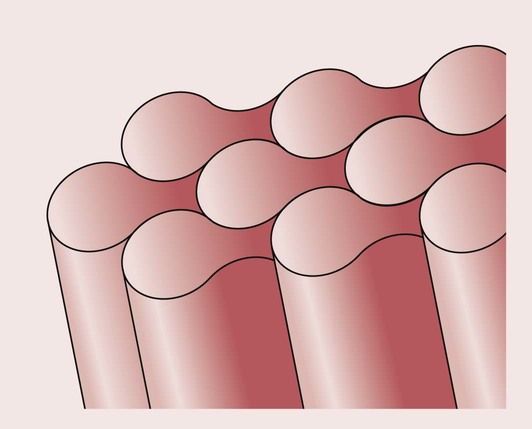
The prisms are long, rod-like shapes of approximately 5 µm in diameter, having a distinctive keyhole cross-section with a head and a tail. The prisms are aligned perpendicular to the tooth surface, as shown in Figure 2.5.1.
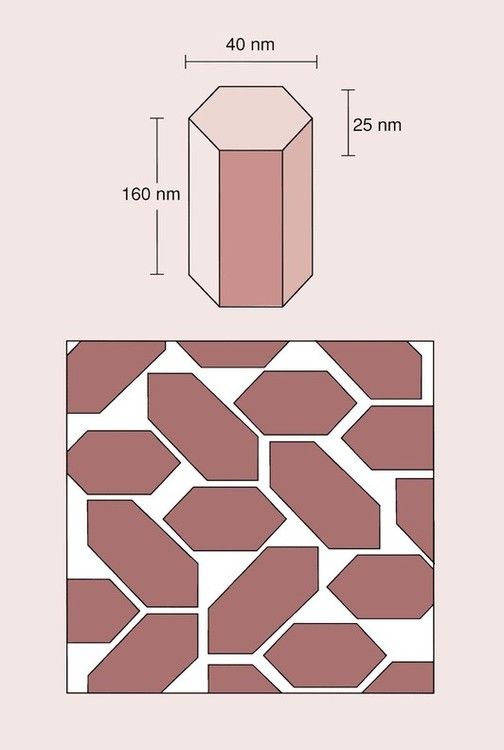
The crystals of hydroxyapatite are flattened hexagonals, as shown in Figure 2.5.2, and, because of their structure, it is not possible to obtain a perfect packing. The spaces left between the crystals are occupied by water and organic material. Much of the water is tightly bound within the enamel structure and not easily removed on drying. The surface layer of enamel tends to have a higher organic content than the deeper layers, and is protected by a layer of pellicle, which is about 1 µm thick.
Acid-etch technique
Due to its composite structure, the surface of enamel can be modified by the application of acid primers. The importance and potential exploitation of this were first appreciated by Buonocore in 1955, when he found that he could make the surface of enamel more amenable to adhesive techniques by modifying it with the application of a solution of phosphoric acid. Thence, the acid-etch technique for the bonding of composite restorative materials to enamel was developed. Its main effect is that of increasing the surface roughness of the enamel at the microscopic level (Figure 2.5.3) and raising the surface energy, which improves wettability (see below).

A major shortcoming of composites is that they have no intrinsic adhesive qualities to tooth tissues, as the resins are essentially non-polar. The acid-etch modification of the enamel surface allows the formation of an intimate micro-mechanical bond between enamel and the resin component of the composite, as long as there is close adaptation at the molecular level.
This discovery has allowed the introduction of a wide variety of restorative techniques that were not previously possible, such as fissure sealants, directly bonded orthodontic brackets, resin-bonded bridges and laminate veneers. Many studies have contributed to our understanding of the relationship between etched enamel and resins, such that now the acid-etch technique forms an integral part of restorative procedures using composites.
As mentioned, the application of a strongly acidic solution (such as phosphoric acid) to enamel has the effect of modifying the surface characteristics. It does this in two important ways.
Clinical procedure
The features of increased surface roughness and raised resin wettability using the acid etching of the enamel surface combine to offer the opportunity for an excellent bond between a composite and the enamel. However, as with any seemingly simple technique, mistakes are easily made unless the operator adheres strictly to the rules for achieving a bond. The various stages of the acid-etch technique can be identified as follows:
Patient selection
The first rule of achieving a good adhesive bond is that the surfaces to be bonded to must be kept free of contaminants. If the surface becomes contaminated with water or saliva, a good bond between the composite and enamel will not be obtained. The highly polar nature of the surface contaminants will prevent the non-polar resin from closely adapting to the enamel surface.
The best approach to the prevention of contamination of the surface of the enamel is to use rubber dam. This may not always be possible, and in those cases where rubber dam cannot be used, it is inadvisable to adopt an adhesive approach.
Appropriate patient selection can avoid these problems. In particular, the use of the enamel acid-etch technique should not be used in patients with mental health issues, children and very elderly or frail patients.
Enamel prophylaxis
As with any other adhesive joint, it is important that the surface of the substrate is thoroughly cleaned. The surface of enamel is covered with a layer of pellicle and possibly a layer of plaque as well. Such layers need to be removed before the etching process.
Whereas a thin layer of pellicle may be stripped off by the acid, it is not possible to remove thick deposits of plaque in this way. If this cleaning is not done, the resin will effectively bond to the surface contaminants and not the enamel. Cleaning of the enamel surface is best performed with a slurry of pumice and water, applied with a bristle brush for some 30 seconds. It is best to avoid proprietary brands of prophylactic pastes, as these may contain components such as oils which are left behind on the enamel surface. These will have the effect of reducing the wettability of the enamel surface by the resin.
Once the surface has been cleaned, it should be thoroughly washed and dried to remove all the debris.
Application of the etchant
Considerable research has been undertaken to evaluate the best method of etching the surface of enamel.
With the teeth dried and properly isolated from the saliva, the aqueous solution of phosphoric acid-etchant can be applied to the enamel with a cotton pledget. An interesting and important observation is that there is an inverse relationship between the etching efficiency and the concentration of the phosphoric acid. High concentrations of phosphoric acid are not as effective at producing the ideal etch pattern as low concentrations. The optimum concentration appears to be in the range of 30–50%. Excessively high concentrations of phosphoric acid tend to show minimal change of the enamel surface, possibly because the low pH causes a rapid saturation of the solution with the reaction by-products, slowing down the rate of dissolution. Hence, the use of phosphoric acid solutions supplied with zinc–phosphate cements should not be used, as the concentration is too high (approximate acid concentration 65%).
It is important that the surface of the enamel is not rubbed during the etching process, as the enamel prisms that stick up from the surface are extremely friable and will break under even the slightest load. A rubbing action will have the effect of breaking all of these prisms, and the crevices and cracks for resin tag formation will be lost.
It is also important that all of the phosphoric acid and the reaction products produced during the etching process are removed. Too often this is dealt with in a cursory and dismissive manner. The procedure to adopt is to wash the enamel surface with copious amounts of water, and to follow this with a water–air spray for no less than 20 seconds. If cotton rolls are used to isolate the teeth, these will have to be replaced in order to ensure a dry field.
The drying process is equally critical, as the objective is to achieve a perfectly dry enamel surface.
The removal of surface hydroxyl groups in the drying procedure will enhance the wettability of the resin on the surface and allow it to flow readily over the surface and into all the little cracks and crevices generated by the etching process. It is important to ensure that the air-hose that is used for drying is free of any contaminants such as oils and water. The etched and dried enamel should have the appearance of a dull, white, slightly frosted surface finish.
Application of unfilled resins
When the resin is applied to a dry and well-etched enamel surface, it will readily invade all the surface irregularities and form resin tags that penetrate the enamel to a depth of up to 30 µm. This produces a very effective bond, by the mechanism of micro-mechanical interlocking.
Although opinions are divided, it is recommended, on balance, that a low-viscosity resin (i.e. either an unfilled Bis-GMA resin or one of the many dentine adhesive resins) is applied to the enamel surface prior to placement of the composite. The rationale for the use of such an intermediate bonding resin is that the low viscosity of the bonding agent facilitates a better penetration into the microscopic spaces in the etched enamel than would be achieved by the direct placement of the composite.
The wettability of the resin composite is as good as that of low-viscosity resin, but the high viscosity of the composite prevents it from spreading easily over the surface of the enamel. Also, the viscosity of the composite is sufficiently high that it can actually bridge across the recesses in the enamel and cause entrapment of air. This has the dual effect of creating a zone of inhibition of the cure of the resin and an interfacial defect, which may be the source of subsequent bond breakdown.
Bond strength
If the above procedure is carried out with diligence, an extremely effective bond between the enamel and the composite is created. In those situations where failure of the adhesive bond has occurred, it can usually be ascribed to poor clinical technique.
Clinically, bonding to enamel should not present a problem. The intimate micro-mechanical interlocking between the etched enamel and the resin will be such that it should be impossible to separate the resin from the enamel without causing the resin or the enamel to fracture. However, this does not mean that failure of enamel bonded restorations will not occur, since cohesive failure of the adhesive or the restoration can still take place. Equally, metallic or ceramic restorations can fail adhesively due to a lack of bonding between the resin and these restorative materials. When a bond failure occurs, it is very important to establish where the fracture has arisen, as this will tell you which component represented the weakest link in the bonding system.
Dentine bonding
Structure of dentine
Dentine is composed of approximately 70% inorganic material, 20% organic material and 10% water by weight (see Table 2.5.1). The inorganic material is mainly hydroxyapatite and the organic material is predominantly collagen. A characteristic feature of dentine is the arrangement of dentinal tubules that traverse its entire thickness. The presence of these tubules makes the dentine permeable to drugs, chemicals and toxins, which can diffuse through the dentine and injure the pulp.
The heterogeneous composition of dentine makes it a particularly difficult substrate to bond to with an adhesive. A second problem is that the differential pressure between the pulp and the dentine floor causes fluid to pump out of the dentinal tubules, such that it is not possible to create a dry dentine surface. Excessive desiccation of the dentine is likely to result in irreversible damage of the vital pulp and is thus not an option.
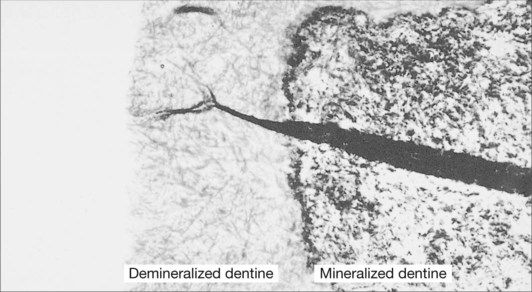
In the case of an abrasion/erosion lesion, the dentine surface usually consists of sclerotic dentine that is covered with a layer of pellicle, plaque and possibly calculus. It is important that these surface contaminants are removed prior to the use of a dentine-bonding procedure. This removal is very readily achieved with the application of pumice and water. A surface is then available that should be free of any contaminants and ready for the bonding procedure. However, the dentine surface is still covered with a layer of disorganized dentine known as the smear layer (Figure 2.5.5).
The smear layer essentially consists of a gelatinous surface layer of coagulated protein, some 0.5–5 µm thick. It is generally highly contaminated with bacteria from the caries process and contains cutting debris. The problems with dentine bonding can thus be summarized as follows:
Components of dentine-bonding agents
Based on the concepts of primers and coupling agents as discussed in Chapter 1.9, dentine-bonding agents can be considered to consist of three essential components, namely:
In the dental literature, the primers are commonly called dentine conditioners, and consist of a variety of acids that alter the surface appearance and characteristics of the dentine. The coupling agents are, in effect, the components that do the bonding, but are generally described in the dental literature and by the manufacturers as primers. The function of the sealer is to flow into the dentinal tubules and seal the dentine by producing a surface layer rich in methacrylates that will ensure bonding to the resin in the composite. This component is variously referred to as the bond, the resin or the adhesive. This last term is especially confusing since it is the coupling agent that provides the bond. This mixing of terminology for the various components of dentine-bonding agents adds to the general confusion surrounding them. In further discussions of dentine-bonding agents we will use the terminology that is commonly used in the dental literature – dentine conditioners, primers and sealers – so as hopefully not to add to the confusion. Besides, later we will discuss how these various components can be mixed up in order to produce formulations that are easier to use.
Role of the dentine conditioner, primer and sealer
Dentine conditioners
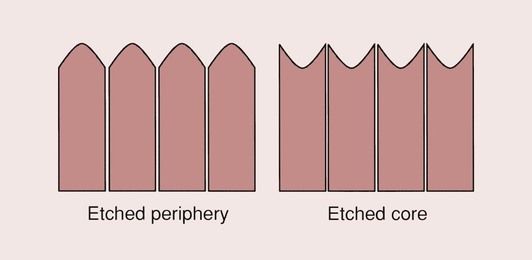
The role of the dentine conditioners is to modify the smear layer that is formed on the dentine due to the cutting action of the bur when preparing a cavity or during exposure to abrasives such as tooth pastes in smooth surface caries and abrasion/erosion lesions. One of the major distinguishing features of dentine-bonding agents is the variety of dentine conditioners that have been used over time. These include maleic acid, EDTA (ethylenediaminetetraacetic acid), oxalic acid, phosphoric acid and nitric acid. What these substances have in common is that they are all acids and they modify the smear layer to varying degrees. The application of an acid to the dentine surface induces an acid–base reaction with the hydroxyapatite. This causes the hydroxyapatite to be dissolved and results in an opening of the dentinal tubules and the creation of a demineralized surface layer of dentine that is generally up to 4 µm deep (Figure 2.5.6). The stronger the acid, the more pronounced these effects. Thus, for EDTA, which is a mildly acidic chelating agent, only partial opening of the tubules occurs (Figure 2.5.7), whereas for nitric acid, which is a strong acid, extensive opening of the dentinal tubules occurs (Figure 2.5.8). The effect of this is shown schematically for a cross-sectional view of the dentine in Figure 2.5.9.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses