Infection Control
Exposure Risks and Effect of Infections on Dentistry
Pervasive increases in serious transmissible diseases over the last few decades have created global concern and have affected the treatment approach of all American health care practitioners. Every health care specialty that involves contact with mucosa, blood, or blood-contaminated body fluids is now regulated. The goal is to ensure compliance with standard precautions and other methods to minimize infection risks.1
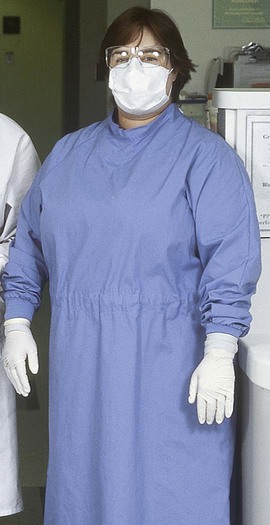
Although the objective of operative dentistry has been to provide the highest standard of care, a prevailing concern has been to minimize the patient’s anxiety with regard to treatment. Providing a supportive, informal, relaxed, and nonthreatening operatory environment has been one emphasis. Although that concern has not waned, emphasis now has expanded to ensuring and showing to patients that they are well protected from risks of infectious disease. Universal use of treatment gloves, masks, protective eyewear, overgarments, plastic barriers to protect equipment, proper disinfectants, and instrument sterilization provides a professional health care atmosphere that conveys conscientious protection and treatment according to sound principles of infection control in keeping with current regulations (Online Fig. 19-1).
Environment of the Dental Operatory
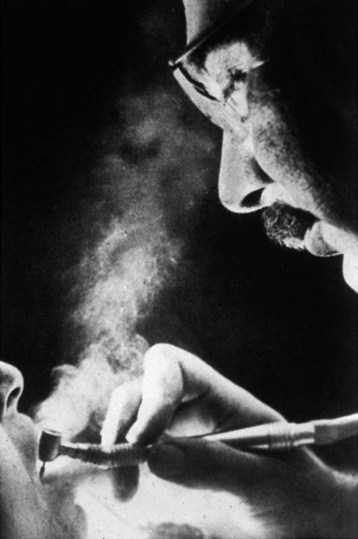
To comprehend the problem of microbial contamination that confronts dentistry, it is necessary to examine the dental treatment environment. Because it was poorly understood in the past, personnel went unprotected from unseen exposures. For most of the twentieth century, general dentistry was routinely practiced without barriers to protect eyes, nose, mouth, and hands as shown in Online Figure 19-2. Not until 1991 were dental personnel required to wear gloves, masks, gowns, and protective eyewear while treating patients. Microbial exposures in the dental operatory include air-borne contamination (see Online Fig. 19-2) and direct and indirect contamination of surfaces.
Air-Borne Contamination
A high-speed handpiece is capable of creating air-borne contaminants from bacterial residents in the dental unit water spray system and from microbial contaminants from saliva, tissues, blood, plaque, and fine debris cut from carious teeth (see Online Fig. 19-2). With respect to size, these air-borne contaminants exist in the form of spatter, mists, and aerosols. Aerosols consist of invisible particles ranging from 5 mm to approximately 50 mm that can remain suspended in the air and breathed for hours.2 Aerosols and larger particles may carry agents of any respiratory infection carried by the patient. No scientific evidence indicates, however, that fine aerosols have transmitted the blood-borne infection caused by hepatitis B virus (HBV).3,4 Transmission of human immunodeficiency virus (HIV) by aerosols is even less likely, as evidenced by the extremely low transmissibility of HIV in dental procedures and in the homes of infected persons.5–8 Mists that become visible in a beam of light consist of droplets estimated to approach or exceed 50 mm. Heavy mists tend to settle gradually from the air after 5 to 15 minutes.9 Aerosols and mists produced by the cough of a patient with unrecognized active pulmonary or pharyngeal tuberculosis are likely to transmit the infection.10 Spatter consists of particles generally larger than 50 mm and even visible splashes. Spatter has a distinct trajectory, usually falling within 3 feet (ft) of the patient’s mouth, having the potential for coating the face and outer garments of the attending personnel.9 Spatter or splashing of mucosa is considered a potential route of infection for dental personnel by blood-borne pathogens.7,11
Barrier protection of personnel using masks, protective eyewear, gloves, and gowns is now a standard requirement for dental procedures. A pretreatment mouthrinse, rubber dam, and high-velocity air evacuation also can reduce microbial exposure.9,11 To help reduce exposure to air-borne particles capable of transmitting respiratory infections, adequate air circulation should be maintained, and masks should be kept in place until air exchange in the room has occurred or until personnel leave the operatory.10
Indirect Contamination
With saliva-contaminated hands, the hygienist, the dentist, and the assistant could repeatedly contact or handle unprotected operatory surfaces during treatments. The invisible trail of saliva left on such contaminated surfaces often defies either awareness or effective cleanup. Soiled surfaces that are poorly cleaned provide another source of gross environmental contamination and thus potential contamination of personnel and patients. Cross-contamination of patients by such contaminated surfaces was documented in a clinical office radiology setting.12,13
Another study used water-soluble red-fluorescent poster paint (plain water-soluble fluorescent-red tempera in water) as a visible substitute for saliva to elevate awareness and facilitate problem solving in infection control. In this study, a hygienist was photographed treating a manikin fitted with dentures coated with red paint (Online Fig. 19-3).14,15 The results showed how extensively the dental operatory surfaces were smeared; how time consuming, expensive, and difficult it was to clean the contaminated surfaces; and how difficult it was to identify, clean, and disinfect objects covered with actual films of invisible saliva. Red poster paint is still used in dramatic training exercises, workshops, and poster displays to show or evaluate contamination control in dental operatories.
Bacterial contamination of dental operatory surfaces was investigated in 10 private dental offices after the surfaces were cleaned and disinfected.14 Sampling confirmed widespread residual contamination with oral bacteria. Contamination was not controlled by conscientious following of cleaning and disinfecting procedures. Items or areas still contaminated after cleaning included handpieces; unprotected lamp handles; air-water syringe handles; control switches on the patient’s chair; tubes, jars, and canisters of treatment materials; seat edges and rests of the dentist’s and assistant’s chairs; faucet knobs; cabinet, drawer, and operatory tray handles; room light switches; and operatory telephones. Telephone handles at the receptionists’ desks also became heavily contaminated with bacteria from saliva. Before handpiece sterilization requirements, contaminated handpieces and other equipment were cleaned only by wiping with disinfectant before reuse. (When nondental offices that were never disinfected were sampled as controls, phone handles and other similar surfaces were devoid of bacteria from saliva.) Amalgam mixing equipment, light-curing units, and camera equipment also are subject to heavy contamination by soiled hands. Maintaining no contamination of these items and areas is a priority objective today. Controlling contamination of equipment and personnel is essential to protecting patients and personnel in this operatory zone of potential heavy contamination. Barrier protection of personnel and equipment, instrument sterilization, and methods of avoiding direct contact with various surfaces are necessary.9,11,14
Patient Vulnerability
Although infection risks for dental patients have not been as well investigated as risks of hospital patients, they seem to be low. Nine cluster cases of dentist-to-patient transmission of hepatitis B virus (HBV) and one cluster case of HIV have been documented since 1971. Since 1986, when infection control practices became widespread, no cluster cases of HBV transmission related to dentistry have been reported.8,16–18
Personnel Vulnerability
When dental personnel experience exposure to saliva, blood, and possible injury from sharp instrumentation while treating patients, they are more vulnerable to infections if they have not had the proper immunizations or used the proper protective barriers. It is unfortunate that the need for proper control of exposures and infections was not realized before the occurrence of the blood-borne HBV infection, which poses a serious threat to all dental personnel (see the section on the impact of HBV).19 HIV has not taken a similar or worse toll, primarily because of the implementation of adequate infection control principles and surveillance. Transmission of occupational disease from the patient to the dental health care worker is low.17 Dental personnel who have treated infectious patients on a daily basis for years in hospital dental services have found infection control methods to be highly effective.20 Infection control has helped dramatically reduce the risks and concerns of personnel in private dental offices and has instilled confidence in a safe environment for patients as well as personnel.
Impact of Hepatitis B Virus
HBV was the first infectious disease to gain attention as a risk for health care personnel who come in contact with blood and other bodily fluids. From 1982 to 1986, various blood sample studies in the United States showed that 14% to 28% of general dentists, 13% of dental assistants, and 17% of dental hygienists had evidence of past infection with HBV.19–23 If only 20% of the approximately 120,000 dentists in the United States had been infected by 1982, 24,000 dentists would have been infected with HBV. With the 2% mortality rate that characterizes HBV, 480 of these infected would have died within 20 to 30 years after initial infection. A vaccine has dramatically curtailed HBV infection among dental personnel who have been effectively immunized. Infection control procedures remain a major concern, however, to prevent cross-infection among patients.11
Impact of Human Immunodeficiency Virus and Acquired Immune Deficiency Syndrome
In view of the high HBV infection rate among dental personnel, epidemiologists anticipated that acquired immune deficiency syndrome (AIDS) would decimate the workforce population in dentistry. By the mid-1980s, HIV had infected approximately one million persons in the United States, most of whom were high-risk persons in metropolitan areas. By 1988, of more than 1000 dentists surveyed in high-risk areas who practiced with unprotected hands, only one was found to be infected, and this person claimed no other exposure risks. As of December 2006, no dentists for whom negative HIV blood tests were established at the time of job-related exposure have acquired job-related HIV infection.1,24–26
Public alarm was intense when a Florida dentist with clinical AIDS transmitted his unique strain of HIV to six patients in his large dental practice.27,28 No other instance of clinician-to-patient transmission of HIV has been documented in dentistry. That isolated instance of HIV transmission contrasts dramatically with the transmissibility of HBV. Twenty reports have documented that more than 300 patients treated by HBV-infected health care workers acquired the virus. Nine of the reports in the United States listed more than 140 patients infected with HBV by dental practitioners that caused several deaths.18,29 Evidence indicates that the Florida cluster of HIV infections and most treatment-related HBV infections from infected clinicians to patients could have been prevented by conscientious use of infection control procedures.17,24 The Florida outbreak was nonetheless tragic for the individuals and families involved. The ensuing public demand for mandatory testing of all health care personnel was reduced to voluntary testing, and states were required to enforce U.S. Public Health Service guidelines for infection control in all health care facilities.30 Public concern continues to focus unprecedented attention on the standards of infection control used in all health care professions, particularly in dentistry.6,30–33
Despite the deficit in patient infection data and the misplaced concern regarding the transmissibility of HIV infection in dentistry, the Florida cluster of HIV infections and Occupational Safety and Health Administration (OSHA) regulations have provided, within a brief time span, a strong impetus to strengthen and control aseptic standards in all health care disciplines.34 Dental students, auxiliary personnel, and patients all are the final beneficiaries of the dramatic changes that have occurred. Infection control is now accepted as a standard of care by dentists.35,36
Federal and State Regulations to Reduce Exposure Risks from Pathogens in Blood and Other Sources of Infection
The term infection control program has a long tradition in hospital usage. Infection control programs such as those recommended by the Centers for Disease Control and Prevention (CDC) and the American Dental Association (ADA) are designed to protect both patients and personnel.37,38
The federal Occupational Safety and Health Administration (OSHA) uses a different term, exposure control plan, for the required office programs designed to protect workers against risks of exposure to infection. Other agencies’ guidelines and requirements that pertain to areas of infection control not covered by the OSHA are discussed in the next section. State occupational safety and health agencies are now enforcing regulations finalized by the federal OSHA, whose Final Rule (or The Standard) on occupational exposure to blood-borne pathogens was published in December 1991.34
The OSHA rule derives from the original Occupational Safety and Health Act passed by the U.S. Congress in 1970.39 This Act identified employers’ obligations to protect employees from occupational risks. The Act has been the basis for all subsequent federal safety and health regulations. According to the Act, each employer must furnish employees with a place and conditions of employment free from recognized hazards that presently cause, or are likely to cause, death or serious harm to employees as specified in the “General Duty Clause” of the OSHA regulations. The Act created the OSHA in the U.S. Department of Labor. In the late 1980s, labor unions petitioned the OSHA in federal courts to extend chemical hazards protection standards to employees in the health care professions. Shortly thereafter, concerns about the transmission of HIV to health care workers stimulated the unions to take similar action to obtain the OSHA regulation with regard to exposure to blood and bodily fluids among health care personnel.
The Act covers two regulated programs of compliance: (1) the OSHA Hazard Communications Program, which deals with risks from environmental and chemical hazards in the workplace, and (2) the OSHA Bloodborne Pathogens Program, which addresses control of “occupational exposure to blood and other potentially infectious materials.”34,40 The OSHA Hazard Communications Program, which also must be implemented in every dental office, applies mainly to chemicals.40
All aspects of the OSHA Bloodborne Pathogens program, which aims to protect employees, were required in every dental office by July 6, 1992.34 Federal Law 42, passed by Congress in 1991, required state public health departments to apply similar standards or follow the CDC guidelines of infection control among all dental care personnel to ensure the protection of patients.30 Under federal and state laws, “employers (including dentists operating nonincorporated offices) must comply with infection control regulations.”
Preparing a Written Occupational Safety and Health Administration Office Exposure Control Plan: Summary
Exposure Control Plan
The OSHA exposure control plan uses terms that require definition. Exposure is defined in the OSHA regulation as “specific eye, mouth, other mucous membrane, nonintact skin, or parenteral contact with blood or other potentially infectious materials (OPIM) that results from performance of an employee’s duties.”41 Only in dentistry is saliva considered a potentially infectious material because oral manipulations and dental treatments routinely cause saliva to become contaminated with the patient’s blood. In dental practice, all patients must be treated with standard precautions to reduce the risk of disease transmission.
Dentists should obtain and read a copy of the Final OSHA Rule on Bloodborne Pathogens to be apprised of complete and exact regulatory details.34 A summary of the current OSHA regulations specifying what employers must furnish, directions employers must provide, and compliance required of employees is as follows:
1. Employers must provide HBV immunization to employees, without charge, within 10 days of employment. The employer also must provide a copy of the OSHA regulations on blood-borne pathogens (from which this information is taken) to the health care professional responsible for providing HBV vaccination.
2. Employers must mandate that standard precautions be observed to prevent contact with blood and other potentially infectious materials. Saliva is considered a blood-contaminated bodily fluid in relation to dental treatments.1,6,7,11,31
3. Employers must implement engineering controls to reduce the production of contaminated spatter, mists, and aerosols. Examples are use of a rubber dam, high-volume suction, rubber prophy cup instead of brushes, scaling instruments for patients with respiratory infections instead of cavitron, and hard-wall containers to avoid contact with disposable and reusable sharps.42–44
4. Employers must implement work practice control precautions to minimize splashing, spatter, or contact of bare hands with contaminated surfaces. Telephones, switches, door handles, or faucet handles should never come in contact with soiled gloves. The subsequent items below (#5–#18) also are work practice control regulations.
5. Employers must provide facilities and instruction for washing hands after removing gloves and for washing other skin immediately or as soon as feasible after contact with blood or potentially infectious materials (Online Fig. 19-4, 19-5, and 19-6). If hands are not visably soiled, cleaning them with alcohol gels is acceptable. The eye or mucosa should be flushed immediately or as soon as feasible after any contact with blood or potentially infectious materials.
6. Employers must prescribe safe handling of needles and other sharp items. Needles must not be bent or cut. When necessary, needles may be resheathed with mechanical aids or other one-handed techniques.
7. Employers must prescribe disposal of single-use needles, wires, carpules, and sharps as close to the place of use as possible, as soon as feasible, in hard-walled, leak-proof containers that are closable, from which needles cannot be easily spilled. Containers must be red in color or bear a biohazard label and must be kept upright and closed when moved. Teeth must not be discarded into trash but can be given to the patient or discarded in sharps containers.
8. Contaminated reusable sharp instruments must not be stored or processed in a manner that requires employees to reach into containers to retrieve them. A basket or cassette should be used to place instruments into, and retrieve them from, soaking pans and ultrasonic cleaners. Biohazard-labeled or red-colored pans that are leak-proof and puncture-resistant should be used.
9. Employers must prohibit staff from eating, drinking, handling contact lenses, and application (but not wearing) of facial cosmetics in contaminated environments such as operatories and cleanup areas. Storage of food and drinks in refrigerators or other spaces where blood or infectious materials are stored should be banned.
10. Blood and contaminated specimens (e.g., impressions that have not been well cleaned and well disinfected, teeth, biopsy specimens, blood specimens, and culture specimens) to be shipped, transported, or stored should be placed in suitable closed containers that prevent leakage. An adequately strong plastic bag can be used for impressions. The surface of all containers must be clean or enclosed in another clean, red, or biohazard-labeled container.
11. At no cost to employees, employers must provide them with necessary PPE and clear directions for use of appropriate universal barrier protection in treating all patients and for all other contact with blood or other infectious materials (see Online Figs. 19-1 and 2-4). PPE must not allow blood or other potentially infectious material to pass through to contaminate personal clothing, skin, or mucous membranes. Employers must provide protective gloves, or hypoallergenic gloves, as needed; appropriate protective body clothing such as gowns, the type and characteristics of which “depend upon the task and degree of exposure anticipated”;34 protective eyewear, chin-length face shields, goggles, or glasses with solid protective side shields; masks; pocket resuscitation masks for cardiopulmonary resuscitation; and surgical caps or shoe covers to be worn when required for surgery or whenever heavy contamination can be reasonably anticipated.
12. Employers should ensure that employees correctly use and discard PPE or prepare it properly for reuse. Adequate facilities should be provided to discard gowns or laundry in the location where they are used. A face shield is not a substitute for a mask.
13. As soon as feasible after treatments, staff should attend to housekeeping requirements, including cleanup of floors, countertops, sinks, and other environmental equipment that are subject to contamination. Housekeeping requirements include the changing of protective covers after each appointment; alternatively, contaminated surfaces and operatory equipment items that cannot be covered should be thoroughly cleaned and disinfected; discarded; or removed and sterilized. (See the sections on operatory asepsis, and procedures, materials, and devices for cleaning instruments before sterilization for details.)
14. Employers must provide a written schedule for cleaning and decontaminating equipment, work surfaces, and contaminated floors. For contaminated spills, an appropriate method of cleaning and the application of disinfecting methods should be prescribed. Broken glassware that may be contaminated must be cleaned with mechanical means and never with gloved hands.
15. Contaminated equipment that requires service first must be decontaminated, or a biohazard label must be used to indicate contaminated parts.
16. Contaminated sharps are regulated waste and should be discarded in hard-walled containers. With regard to OSHA requirements in dentistry, regulated waste also means (1) liquid or semi-liquid blood or other potentially infectious materials, (2) contaminated items that would release blood or other potentially infectious materials in a liquid or semi-liquid state if compressed, and (3) items that are caked with blood or other potentially infectious materials and are capable of releasing these materials during handling. Such regulated waste should be disposed of properly in biohazard-labeled or red-colored closable bags or other labeled containers that prevent leakage. Containers contaminated on the outside must be placed in a secondary container. The secondary container also must be closable, prevent leakage, and be red-colured or biohazard-labeled. Containers or bags must be closed when moved. If outsides of reusable containers are likely to become contaminated, they must be inspected, decontaminated, and cleaned on a regularly scheduled basis and as soon as feasible if they become visibly contaminated. Cabinets or other storage areas on the premises in which blood-contaminated waste is stored must be identified by a biohazard label.
17. Reusable contaminated sharp instruments should be placed in a basket in a hard-walled container for transportation to the cleanup area. Personnel must not reach into containers of contaminated sharps.
18. Employers must provide laundering of protective garments used for standard precautions at no cost to employees. Contaminated laundry should be handled as little as possible without sorting or rinsing. All soiled linens should be bagged where they are used in a color-coded bag clearly indicating requirement of universal precautions.
Emergency and Exposure Incident Plan
1. Exposures to mucosa may not be associated with an injury, or an exposure incident may involve minor or severe injury (e.g., from a cutting instrument). Rapid and thorough cleaning of a wound or washing a splashed eye or mouth as quickly as possible is the most important first step to minimize infection risks. Blood tends to collect on the surface of puncture wounds created by solid pointed instruments, so washing puncture wounds is just as important. Specific staff members to provide any help, direction, or transportation needed to obtain medical care must be identified. A brief written plan for accessing rapid medical attention should be formulated. This content should constitute the first part of the exposure incident plan. Sufficient time will still be available for a designated responsible individual to contact the patient and transmit medical records and other information to the attending physician, as presented next.
2. The written permission of the patient who is the source of exposure must be obtained to copy and convey his or her medical history to the attending physician or to obtain other medical records regarding the patient. Knowledge of risk behavior, blood test results, or other pertinent information usually can be conveyed verbally in confidence, however, without permission in case of exposure. Local laws must be consulted. Some states only prescribe communication of the name, address, and phone number of the patient and the name and phone number of the patient’s physician to the attending physician of the exposed individual. The examining physician will contact the patient’s physician, who will then deal with testing the patient.
3. As directed by OSHA regulations, employers must provide a copy of the exposure incident plan and explain it to the employees. Employers must document the route and circumstances of the exposure, identifying the source patient when possible. Employers must provide and pay for exposure incident evaluation and follow-up evaluations for an exposed employee, or these may be paid for by workers’ compensation.
4. If other local regulations do not exist, employers also must (a) identify and contact the source patient if possible; (b) obtain the source individual’s permission to be tested, unless he or she already is known to be infected; (c) have the source individual’s blood tested by a health care professional, as soon as feasible, for evidence of current HIV or HBV infection (e.g., if blood is available, some states permit testing without permission in exposure instances); (d) provide results to the exposed employee in confidence (state laws often require counseling of the source patient and the exposed individual for HIV testing); (e) test the employee’s blood, with his or her permission, as soon as feasible; (f) hold any available sample of the employee’s blood for 90 days if consent is not given for HIV testing to provide for any change of mind; and (g) provide post-exposure prophylaxis to the employee, when medically indicated, according to recommendations of the U.S. Public Health Service.
5. The attending physician must be provided with a copy of OSHA regulations (from which this information is taken), documented information regarding the incident, results of the source individual’s tests, and the employee’s immunization records and any other relevant medical records.
6. A written report from the attending physician must be obtained by the employer and provided to the employee within 15 days of the completion of evaluation, stating that the employee has been informed of the results, possible infection consequences, and any further evaluation or treatment needed that relates to the exposure incident. Unrelated diagnoses or findings remain confidential.
Training of Personnel Required by Occupational Safety and Health Administration
Occupational safety guidelines require that new office personnel who will have contact with blood and blood-contaminated body fluids receive initial training in infection control. Re-training is required annually and whenever the exposure control protocol changes.34 Training of personnel must contain the following elements, as listed in the OSHA standard:
1. An accessible copy of the regulatory text of this standard and an explanation of its contents
2. A general explanation of the epidemiology and symptoms of blood-borne diseases
3. An explanation of the modes of transmission of blood-borne pathogens
4. An explanation of the employer’s exposure control plan and the means by which employees can obtain a copy of the written plan
5. An explanation of the appropriate methods for recognizing tasks and other activities that may involve exposure to blood and other potentially infectious materials
6. An explanation of the use and limitations of methods that would prevent or reduce exposure, including appropriate engineering controls, work practices, and PPE
7. Information on the types, proper use, location, removal, handling, decontamination, and disposal of PPE
8. An explanation of the basis for selection of PPE
9. Information on the HBV vaccine, including information on its efficacy, safety, method of administration, benefits of being vaccinated, and that the vaccine and vaccination will be offered free of charge
10. Information on the appropriate actions to take and persons to contact in an emergency involving blood or other potentially infectious materials
11. An explanation of the procedure to follow if an exposure incident occurs, including the method of reporting the incident and the medical follow-up that would be made available
12. Information on the postexposure evaluation and follow-up that the employer is required to provide for the employee after an exposure incident
13. An explanation of the signs and labels or color coding required by the OSHA standard
14. An opportunity for interactive questions and answers with the individual conducting the training session34
15. Additional specific information must be provided regarding the details and cleanup schedules for employees’ operatory and facilities.
Occupational Safety and Health Administration–Required Records
Job classification and immunization and medical records of personnel must be kept for 30 years by the office or a designated physician for OSHA inspection or disposed of, according to requirements. Training records must be kept for 3 years from the date of training. Exposure incidents must be tabulated and posted according to OSHA requirements. Details are provided in the regulations.34 An interpretation of these regulations for dentistry has since been published.45 Some variations from these and other OSHA regulations may be specified later for dentistry as a result of petitions made by the ADA. The dentist should consult current information.
Regulations of Other Agencies
State public health services and dental licensing boards complete the spectrum of infection control regulatory agencies. Most agencies specify the infection control guidelines of the ADA and the CDC of the U.S. Public Health Service, but focus more on tasks and procedures necessary for patient protection in dentistry.5,11,46–48
Regulations Regarding Infected Health Care Personnel
Concerns about the possible transmission of AIDS from infected health care personnel to patients has led the U.S. Public Health Service to recommend additional precautions. All health care personnel who perform invasive, exposure-prone treatments are urged to obtain testing for HBV and HIV infections voluntarily.32
Exposure-prone procedures include simultaneous use of the operator’s fingers and sharp instrumentation in a highly confined or poorly visualized anatomic site such as the mouth, where tissues are cut or bleeding can occur. Clinical personnel are considered infected when they test positive for antibodies against HIV or for hepatitis B surface antigen (HBsAg) and hepatitis Be antigen (HBeAg). Infected health care personnel are advised not to perform exposure-prone procedures unless they have sought counsel from an expert review panel and have been advised under what circumstances they may continue to perform these procedures, depending on the experience and skill of the clinician involved. As defined by the CDC, a review panel may consist of the worker’s physician, an infectious disease specialist with expertise in the epidemiology of HIV and HBV transmission, another health care professional with expertise in the type of procedures performed, and a local public health official.32
Occupational Safety and Health Administration—Required
Acquired Immune Deficiency Syndrome and Human Immunodeficiency Virus Infection
AIDS is the last stage of a debilitating, eventually fatal human disease. AIDS may develop in 1.5 to 11 or more years after an initial infection with HIV.37,49 HIV is a relatively fragile ribonucleic acid (RNA) retrovirus, which is easily destroyed in the dry state in 1 to 2 minutes by most disinfectants.6,7,37,50
Human Immunodeficiency Virus: Epidemiology and Transmission
Since its recognition in 1981, as of the end of 2006, HIV had infected 1.1 million people in the United States, with 21% going undiagnosed.8,33,51,52 HIV is transmitted mainly through blood, blood-contaminated bodily fluids such as semen, and vaginal fluids. High-risk behaviors or situations that define high-risk groups include having multiple sex partners of the same or opposite sex; having a sexual partner who is at high risk or infected; intravenous drug abuse; treatment for hemophilia; blood transfusion received before spring 1985; and infants of an infected mother.33,53–55 Casual, nonsexual contact, including social kissing and sharing towels or food among family members in a household with an AIDS patient, has not been shown to transmit the infection.
Progression of Human Immunodeficiency Virus Infection into Acquired Immune Deficiency Syndrome
After a prolonged quiescent state of 1.5 to possibly 11 years after infection, HIV begins to destroy cells that control the normal immunity of the body against infections and tumors. At that time, the body becomes more and more vulnerable to many common viruses and microbes found in the normal environment. Commonly harmless parasites and fungi are able to cause severe and often fatal conditions such as pneumonia and cerebral infections.37,49
On entering the blood or tissues, HIV can attach only to certain docking sites that it finds projecting from the surfaces of certain white blood cells. Helper lymphocytes crucial to the normal functioning of the immune system are covered with these sites. Immunologists have labeled these cells T helper lymphocytes because the thymus has an important role in preparing them to function. The surface attachment sites are termed category designation four (CD4) glycoprotein antigens. When it becomes attached, virus RNA can enter and infect the lymphocyte.56
The cells commonly infected, termed T4 (CD4) helper lymphocytes, are crucial to normal cellular and antibody functions that protect humans against many bacteria-infected, fungi-infected, and virus-infected cells and tumors or cancers. Other cells such as macrophages, neurologic glial cells, colon or rectal cells, and possibly some connective tissue cells also have the CD4 glycoprotein surface sites to which HIV can attach itself. Colon cells (e.g., in the case of male homosexual intercourse) may serve as infection sites. It is unknown whether the mucosal cells of other body cavities may serve as initial infection sites as well. Various susceptible cells and perhaps the cells in bone marrow may serve as reservoirs of the virus in a prolonged latent or quiescent incubation stage when HIV sometimes cannot be detected in blood.56
HIV is termed an RNA retrovirus, which needs complementary DNA formed within the nucleus of a host cell (termed provirus form) to reproduce the HIV. As HIV gains entry into the lymphocytes, reverse transcription of viral RNA begins, resulting in the formation of double-stranded viral DNA in the infected cells. When inserted into the cell’s genetic structure (genome), this DNA becomes the provirus of HIV. The DNA of HIV may divide and reproduce along with the cell’s nuclear DNA for years. Antibody tests are now available to detect the provirus DNA fragments that regulate the production of various parts of the HIV structure (i.e., core proteins, gag; viral envelope, env; reverse transcriptase, pol).53,56
After remaining latent during the prolonged incubation period in infected helper lymphocyte cells, HIV begins to replicate. The lymphocytes die, releasing the virus into blood, and the numbers of essential helper lymphocytes are drastically reduced. When helper cell counts decrease to less than 200/mm3 in blood, many different opportunistic infections and tumors appear. Conditions are such that it becomes increasingly difficult to treat the patient until fatal Pneumocystis infection of the lungs occurs, or until HIV or other infection of the brain causes death.49 Levels of virus in blood usually increase at this time but are still low compared with the huge viral concentrations reached in the blood of patients with HBV.53 At our institution, patients with T4 helper cell counts of 200/mm3 or less benefit from the protective facilities, nursing care, and treatment expertise offered by the hospital dental service clinicians.
Symptoms and Oral Manifestations
Within 3 months of infection, temporary flu-like symptoms—pharyngitis, myalgia, fatigue, fever, or diarrhea—may occur when antibodies to HIV become detectable. Following the prolonged incubation of the virus for approximately 1.5 to 11 years, any of several early signs of AIDS that signal the progressive failure of the immune system may be observed by the dentist.37,57 During examination, the dentist can easily detect one or two cervical lymph nodes, especially below the mandible, that persist for more than 3 months. The nodes may be attached and painless, or they may be movable, painful, and infected. Undifferentiated non-Hodgkin’s lymphoma may arise in lymph nodes or may appear in the mandible, central nervous system, eyes, bone marrow, and other vital organs.37
Persistent oral candidiasis is often seen with easily dislodged, white, curd-like patches scattered over the tongue. In AIDS, such infection may not respond easily to treatment and often recurs, developing into atrophic candidiasis or cheilitis at the angles of the lips. Painful herpes stomatitis also is common. Untreated herpes or candidiasis may progress to esophagitis or laryngitis, impairing speech.37
Red, brownish-to-purple blotches that persist on the oral mucosa and skin typify sarcoma of the capillaries, termed Kaposi’s sarcoma. Oral lesions often develop into tumors that may require surgery and radiation therapy. Kaposi’s sarcoma often is found on the oral tissues of homosexual men. Human papillomavirus (HPV) can cause oral warts that appear flat or cauliflower-like.37 Persistent, severe, recurrent gingivitis and periodontitis that bring patients to dental care are common findings typical of AIDS. The gingivitis may persist despite effective plaque control.58
Early systemic signs of illness progressing to AIDS are marked by weight loss of 50 lb within a few months and chronic fever or night sweats that persist for 3 months or more.37,49 Early detection and medical treatment of HIV infection is beneficial to most patients. Current treatments are summarized in the annual Journal of the American Dental Association supplement update, Facts about AIDS for the Dentist.37
Serology of Human Immunodeficiency Virus Infection
HIV infection is detected with blood tests (enzyme-linked immunosorbent assay [ELISA], Western blot test, and fluorescent antibody test) that detect antibodies formed against the virus. Tests for anti-HIV antibodies are often positive within 3 months after infection. Most are positive by 6 months; in 1% of cases, it takes 12 months to obtain a positive test. A second positive test is necessary to confirm positive serologies. Serologic tests for the virus and provirus DNA also have been developed. Tests for T4/T8 (or CD4/CD8) lymphocyte ratios are used to identify the progress of the HIV infection. One criterion for starting zidovudine therapy is a T4 helper cell count less than 500/mm3 of blood.37
Human Immunodeficiency Virus Risks for Clinical Personnel
Of all American health care workers injured by needles and sharp instruments used to treat HIV-infected persons, only 0.3% or less have become infected with HIV. This statistic contrasts with 30% of workers who become infected with HBV after parenteral exposure to infected blood.6 As of December 2006, among all U.S. health care personnel, documented occupation-related HIV infections total 57, of which none was reported among dental personnel.1,8,16,17,59 An additional 139 HIV infections are considered possible occupational transmissions, including 6 in dental personnel.1,25
As was pointed out at the beginning of this chapter, dental personnel have been spared, almost miraculously, being infected with HIV. Thousands of unprotected dentists who unknowingly treated HIV-infected patients must have been exposed to HIV as the epidemic mounted during the 1980s before gloves and other barriers came into common use. Only six dentists who claim no other exposure risks seem to have acquired HIV infection by occupational exposure.1,6,8,16,17,25 Testing at the time of exposure for evidence of prior HIV infection was not commonly performed in dentistry until the 1990s. Because none of the infected dentists had such baseline blood tests, their HIV infections cannot be linked firmly to the time and circumstance of clinical exposure.
HIV infection was reported to have developed in a nurse and a technician who were spattered with HIV-infected blood. Other medical personnel have been reported to have acquired HIV infections related to spatter of infected blood on their nonintact skin. The serologic status of HIV in these persons was apparently not known when they were exposed.6,8,16,17 Personnel are required to protect their eyes, mucosa, skin, and hands from spatter and direct contact with blood and blood-contaminated bodily fluids during dental treatments of all patients.45 Precautions also must be taken to minimize risks of injuries with sharp instrumentation.
Patients seriously ill with AIDS who are seen in a hospital setting also may harbor transmissible respiratory infections such as tuberculosis and cytomegalovirus (CMV) infection.60,61 As indicated in the section on the epidemiology of other infection risks, transmission of drug-resistant tuberculosis from immunocompromised patients is a growing concern. Personnel without adequate barrier protection should avoid exposure to coughing, saliva spatter, and heavy aerosols from HIV-infected persons with signs of respiratory infection. This applies especially to pregnant women because recent infection with CMV can be detrimental to the fetus. CMV is also a blood-borne pathogen.
Human Immunodeficiency Virus risks for Dental Patients
With proper use of infection control measures in dental practice, the risk for a dental patient of contracting HIV from office personnel or from other patients is extremely low. HIV has not been transmitted to dental patients from infected clinical personnel anywhere in the United States, with the exception of one unique outbreak.17,24,27 In a circumstance that has been unique as of 2011, six patients were found to be infected with the same strain of HIV present in a Florida dentist who had treated them.17,24,27 These patients had no apparent source of exposure other than the dentist who, in spite of having AIDS symptoms, continued to treat patients. This dentist’s use of adequate infection control measures was questionable. It is quite likely that some kind of clinician-to-patient transmission had occurred in this case. At this time, no other instances of transmission of HIV from infected dentists or physicians to patients have been reported. One or more alleged HIV cross-infections between patients, attributed to contaminated dental equipment, are under investigation.16
Human Immunodeficiency Virus Data Related to Infection Control
Data that provide a better understanding of disease agents, their survival qualities, and clinical transmission potentials help clinicians institute effective infection control. The following HIV data are reassuring and help explain the amazingly low occupational risk of HIV infection for dental personnel:26,37
1. In contrast to HBV, very low levels of HIV usually have been found in the blood of infected persons. This is especially true of asymptomatic persons, who are the most difficult to recognize and would be most likely to be treated in private clinics.31,62
2. HIV was detected in only 28 of 50 samples of blood from infected persons. In saliva from infected persons, HIV was detectable in only 1 of 83 samples.3 Counts of virus per milliliter of blood fluctuate but may increase as the number of antibodies to the HIV core protein decline.53,62
3. CDC investigators have found 99% of HIV to be inactive in approximately 90 minutes in dried infected blood.31 Longer survival data on larger numbers of HIV grown in laboratory cell cultures have created misleading information about the survival of HIV in dried infected blood. In blood that remains wet, however, the virus may survive for 2 or more days.63 Caution is required when handling containers of used needles in which virus-infected blood may remain wet.
4. HIV is killed by all methods of sterilization. When used properly, all disinfectants, except some quaternary ammonium compounds, are said to inactivate HIV in less than 2 minutes.31,37,64
5. HIV has been transmitted through blood-contaminated fluids that have been heavily spattered or splashed on persons.27 Aerosols such as those produced during dental treatments have not been found to transmit HBV or HIV infection.31,65
6. Barriers have proved successful in protecting dental personnel in hospital dentistry and in all other dental clinics against HIV; at our institution, for more than 10 years, they have been providing effective prevention of even more easily transmissible viral infections.
A more recent concern for immunocompromised individuals and for dental personnel is airborne transmission of multidrug-resistant Mycobacterium tuberculosis.10,46,60
Viral Hepatitis: Agents, Epidemiology, and Infection
In the 8 years after AIDS was recognized, 38,000 persons were identified to have developed the disease. During that same period, an estimated 38,400 persons died from HBV, related cirrhosis, or liver carcinoma.33,66,67 Infective inflammation of the liver, termed hepatitis, can be caused by infection from various hepatitis viruses labeled A to G. The type of infection is diagnosed specifically by serologic testing. Hepatitis types A, B, and C are roughly equally divided among cases of viral hepatitis detected in population surveys, with hepatitis A virus (HAV) being the most prevalent. HBV, HCV, and HDV are blood-borne infections. HAV and HEV are fecal-borne infections.67,68 A new blood-borne virus, HGV, has been detected in a group of high-risk hospitalized dental patients with liver disease associated with other viral agents or conditions.69 The importance of HGV and its contribution to liver disease are unclear.
HBV is found in 1 in 100 to 500 persons in the general population (estimated 1.2 million people with chronic infection in the U.S.), including dental patients. The incidence has peaked in areas associated with high rates of intravenous drug abuse and closely follows the incidence of HIV infection.7,42,51,70 According to the CDC, 1 in 55 persons (1.8%) in the U.S. population may carry HCV, with an estimated 3.2 million people with chronic HCV infection.51,68,71,72 HCV accounts for one third of liver transplantations and more than 8000 deaths per year.71
Viral Hepatitis Infection: Symptoms, and Clinical Findings
HBV must enter the circulating blood to reach the liver, where the viral DNA causes infected hepatic cells to reproduce the virus. Symptoms usually appear after 2 to 4 months of incubation. Extensive liver damage and illness occur rapidly in approximately 2 of 10 infected persons. Symptoms and signs include nausea, vomiting, chronic fatigue, mental depression, fever, joint pain, darkened urine, jaundice, elevated liver enzymes, and possibly diarrhea or rash. Mortality is 2% or less but tends to be 2% or greater in individuals older than 30 years of age.29,38 CMV and Epstein-Barr virus (EBV) infections also may produce jaundice and elevated liver enzymes.
Other types of hepatitis produce symptoms similar to those of HBV.22,29,67,68 HAV has a shorter incubation of approximately 1 month and lower mortality. Individuals infected with HAV do not remain infected or infectious beyond 8 weeks after symptoms subside. HCV is often (75%) anicteric (without jaundice), and elevated levels of liver enzymes and serologic tests help establish the diagnosis. HCV becomes chronic in 75% to 85% of the infected individuals, causing them to remain infectious.51,71
HDV, or delta hepatitis virus, has a curious makeup. It has no outer coating and relies on the cells infected with HBV to provide the required outer layer. When HBV and HDV infect an individual concurrently, usually by the same route and source, the infection becomes much more severe and many times more fatal than infection with HBV alone. Protection against HBV also protects against HDV, but not HAV, HCV, or HEV.66–68
Transmission of Viral Hepatitis
The transmission of HBV, HCV, and hepatitis D virus is mainly through blood, intravenous drug abuse, and sexual contact. Billions of HBV may be present in one milliliter of infectious blood.6 HBV also is found in saliva, but at lower concentrations. HBV can be transmitted through contamination of broken skin, the mouth, or the eyes with blood-contaminated saliva. One in three nonvaccinated exposed persons may be infected with HBV. In studies performed during dental treatments of HBV-infected individuals, aerosolization of HBV could not be detected with tests for HBsAg.65
HBV is transmitted in the population through the same routes as those for HIV infection. In contrast to HIV, however, HBV has been transmitted to family members through prolonged associations that may involve repeated contamination with saliva or blood (e.g., through sharing of shaving instruments, traces of blood left on bathroom towels, continuous sharing of unwashed toothbrushes, or drinking from the same cup). In public situations, neither HIV nor HBV is transmitted through casual contact.49,67 Individuals at risk for HIV infection also are more likely to be carriers of HBV. Of HIV-infected individuals, 90% have been infected with HBV. HAV is excreted from the infected liver into bile. HAV and HEV are transmitted by the fecal–oral route. Poor hygiene and contaminated food and water are common routes of infection. These types, however, are not a major concern in dentistry.
Blood transfusions were a major source of HBV infection until 1985 and of HCV infection until 1991. A test for HCV was developed in 1990. Tests instituted in hospitals since 1986 for HBV and since 1991 for HCV have virtually eliminated transfusions as a source of infections. A persistent problem is the detection of infectious donors during the incubation period of the pathogens.68,71
Hepatitis B and Hepatitis C Virus Infection Risks for Personnel
Personnel can be infected through parenteral exposure; mucosal exposure to infected blood or blood-contaminated saliva; and spatter of infected blood to the eyes, mouth, or broken skin.6 Paper cuts from blood-contaminated request forms have been reported to have transmitted HBV.73 Plain saliva also can be weakly infectious. Aerosolized, blood-contaminated saliva and respiratory secretions that can transmit many respiratory viruses and tuberculosis have not been shown to transmit HBV.9,10,42,67,74 One in three parenteral exposures of nonvaccinated personnel to HBV-infected blood has resulted in HBV infection.29 In contrast to the 1 of 300 nonvaccinated individuals who develop HIV after parenteral exposure to HIV-infected blood, 100 of 300 individuals parenterally exposed to HBV develop HBV.
A vaccine against HBV is available. Mortality rates from HBV exposure could approach zero for dental personnel.67 Patient protection still depends on the effective use of infection control procedures.
HCV exposure risks for dental personnel have been documented and appear to be low.75 Infection control should minimize risks. Data indicate that infection rates from parenteral exposure to HCV-infected blood fall between the rates for HBV and HIV infection—approximately 1.8%.71,72,75
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses