Chapter 18
Elastic Impression Materials: Hydrocolloids
18.1 Introduction
Hydrocolloid impression materials used in dentistry are based on colloidal suspensions of polysaccharides in water. A colloidal suspension is characterised by the fact that it behaves neither as a solution, in which the solute is dissolved in the solvent, nor as a true suspension, in which a heterogeneous structure exists with solid particles being suspended in a liquid. The colloidal suspension lies somewhere between these two extremes, no solid particles can be detected and yet the mixture does not behave as a simple solution. The molecules of the colloid remain dispersed by nature of the fact that they carry small electrical charges and repel one another within the dispersing medium. When the fluid medium of the colloid is water it is normally referred to as a hydrocolloid.
Dental hydrocolloid impression materials exist in two forms: sol or gel form. In the sol form, they are fluid with low viscosity and there is a random arrangement of the polysaccharide chains. In the gel form, the materials are more viscous and may develop elastic properties if the long polysaccharide chains become aligned. Alignment of the polysaccharide chains as fibrils which enclose the fluid phase normally causes the gel to develop a consistency similar to that of jelly. The greater the concentration of fibrils within the gel the stronger the jelly structure will be. This point is best illustrated by consideration of the properties of commercial, flavoured gelatin (jelly). The material which is initially purchased is a fairly strong gel but after dilution with water the resulting gel is much weaker. This is relevant to dental hydrocolloids since the strength of the gel is important and depends on the concentration of polysaccharide material dispersed in the aqueous phase.
The conversion from sol to gel forms the basis of the setting of the hydrocolloid impression materials. The products are introduced into the patient’s mouth while in the fluid, sol form. When conversion to gel is complete, and elastic properties have been developed, the impression is removed and the model cast.
The formation of gel and development of elastic properties through alignment of polysaccharide chains may take place by one of two mechanisms. For some materials, gel formation is induced by cooling the sol. Chains become aligned and are mutually attracted by Van der Waals forces. Intermolecular hydrogen bonds may be formed between adjacent chains, enhancing the elasticity of the gel. On reheating the gel, these bonds are readily destroyed and the material reverts to the sol form. These materials are the reversible hydrocolloids (agar). The principle of gel formation is given in Fig. 18.1.
For other materials, gel formation involves the production of strong intermolecular cross-links between polysaccharide chains. These materials do not require cooling in order to encourage gel formation and once formed the gel does not readily revert to the sol form. These materials are the irreversible hydrocolloids (alginates).
18.2 Reversible hydrocolloids (agar)
These materials are normally supplied as a gel in a flexible, toothpaste-like tube or syringe. The gel consists primarily of a 15% colloidal suspension of agar in water. Agar is a complex polysaccharide which is extracted from seaweed. Figure 18.2 gives a very simplified indication of the type of molecular structure. The high molecular weight, coupled with the large concentration of free hydroxyl groups, renders the material suitable for hydrocolloid formation.
Small quantities of borax and potassium sulphate are normally present in the gel. Borax is added to give more ‘body’ to the gel, although the mechanism by which this is achieved is unclear. Unfortunately, borax retards the setting of gypsum model and die materials and models formed in agar impressions may have surfaces of poor quality. The presence of potassium sulphate in the agar gel counteracts this effect of the borax, since it accelerates the setting of gypsum products (see p. 37). Alternatively, the impression may be dipped in a solution of accelerator.
Fig. 18.1 Diagram illustrating the formation of an aqueous polysaccharide gel by ordering of the polymer chains. (a) Disordered chains (present in sol). (b) Ordered chains (present in gel). Chemical cross-links are formed in irreversible materials.
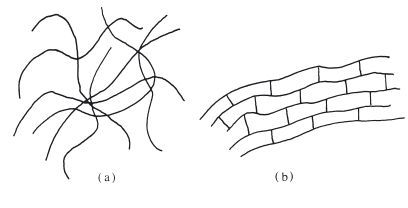
Fig. 18.2 Simplified structural formula of a polysaccharide chain similar to that used in agar.
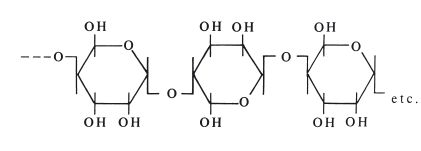
Fig. 18.3 A specialized water bath used for conditioning agar impression material.
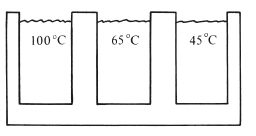
Manipulation: Reversible hydrocolloids are normally conditioned, prior to use, using a specially designed conditioning bath. This consists of three compartments each containing water (Fig. 18.3).
The tube or syringe of gel is first placed in the 100°C bath. This rapidly converts the gel to sol and the contents of the tube become very fluid. The tube is then transferred to the 65°C bath where it is stored until required for use. This temperature is high enough to maintain the material in the sol form. At this stage, the material is mixed by squeezing the tube, thus ensuring an even distribution of components. A few minutes before the impression is recorded, the contents are cooled to 45°C. If the material is maintained at this temperature for long, it slowly begins to revert to the gel form. When the impression is recorded, the sol is expressed from the tube into an impression tray and seated in the patient’s mouth. Reversible hydrocolloids are available in a variety of viscosities to help us achieve high levels of accuracy for use in crown and bridgework. A high viscosity sol is transferred from the tempering bath into a stock tray and a low viscosity material can be syringed directly onto the prepared teeth. The tray is then inserted into the mouth over the teeth concerned. There is a temperature hysteresis effect on the gel to sol and sol to gel transition in that the latter process occurs at a lower temperature than the gel to sol transition. The conversion from sol to gel takes place slowly at mouth temperature and it may be many minutes before the material develops sufficient elasticity to permit removal of the impression. The rate of conversion of sol to gel may be accelerated by spraying cold water onto the impression tray whilst it is in the mouth, or by using water-cooled impression trays. The latter are metal stock trays with a narrow-bore metal tube attached to the outer surface. The tube is connected to a cold water supply and the circulating water reduces the temperature of the tray. The coolest areas of the sol are converted to gel more rapidly, so the material in contact with the tray sets more rapidly than that in contact with the oral tissues. It is argued that this arrangement may be advantageous. If slight movements of the impression tray take place during setting, the material adjacent to the oral tissues can flow to compensate, thus reducing inaccuracies.
Removal from the mouth is accomplished with a snapping action. The reversible hydrocolloids are very susceptible to water uptake and loss (syneresis and imbibition). After recording an impression it should be rinsed to remove debris and then stored covered in a damp gauze. The model should be poured within 30 minutes of the impression being recorded. It is not possible to use a hydrocolloid impression to make metal coated or epoxy resin dies.
One clinical advantage of the reversible hydrocolloids relates to their ability to take up moisture. A poorly fitting provisional crown can result in gingival inflammation and an increased rate of crevicular fluid flow. In turn this makes the recording of an accurate impression of the crown margins more difficult. When a reversible hydrocolloid is used in such patients it tends to ‘draw’ moisture from the marginal gingival tissues. This has the effect of producing a relatively poor first impression but a greater chance of success with the second as the levels of fluid flow will be decreased. It is possible to re-use reversible hydrocolloids. However, concerns about cross-infection control and alteration to the material’s physical properties by altered water content and the incorporation of small chips of dental stone into the material during repeated use make this approach unacceptable.
Properties: Many of the important properties of agar impression materials are embodied in the ISO Standard for dental aqueous impression materials based on agar, ISO 1564. This standard classifies materials according to consistency as:
| Type 1 | high consistency |
| Type 2 | medium consistency |
| Type 3 | low consistency |
Some of these products can be used alone to record impressions whilst others are designed to be used in techniques requiring two materials of different consistency. For example, the type 1 material can be used for making impressions of complete or partial dental arches with or without the use of syringe-extruded increments of type 2 or 3 material. When type 1 is used in combination with types 2 or 3 the type 1 material is softened and extruded into the impression tray whilst the type 2 or 3 material is extruded from a syringe into the mouth to cover the area which is to be recorded.
The type 2 materials are multi-purpose in application as they can also be used for making impressions of complete and partial dental arches with or without the use of a syringe-extruded increment, but these products can also themselves be syringe-extruded for use in the combination technique. The type 3 materials are designed specifically for syringe use and are used with a type 1 or type 2 material in the combination technique. Table 18.1 gives some of the requirements of type 1 and type 2 materials compared with alginate materials. In the sol form, agar is suffic/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


