Chapter 17
Endodontic Microsurgery or Dental Implants?
INTRODUCTION
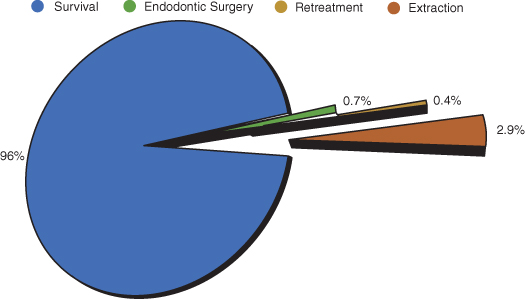
Nonsurgical root canal treatment has advanced over the past two decades in both materials and technique. This improvement has allowed practitioners to approach teeth with a complex root canal system efficiently and provide patients with much more predictable treatment. In year 2000, approximately 30,000,000 endodontic treatments were provided compared to 910,000 dental implants (Millennium Research Group and ADA; Figure 17.1). In the United States, evaluation of 1.4 million teeth with initial endodontic treatment resulted in a 97% survival rate over a period of 8 years. Only 0.7% of treated teeth required surgical root canal treatment. This is in part due to the high survival rate of nonsurgical root canal treatments (Salehrabi and Rotstein 2004; Figure 17.2). On the other hand, dental implants have also advanced surgically and prosthetically, promising highly aesthetic and functional results. Unfortunately, dental implants have been over-commercialized at the expense of other, highly advanced, predictable treatment modalities, namely, endodontic microsurgery. This has resulted in the overall trend of extracting teeth with failed root canal treatments and replacing them with implants instead of referring them to specialists for surgical management. The past experience with the traditional endodontic surgery and the lack of knowledge about the recent advances in the microsurgical approach reinforced this trend.
Figure 17.1 Pie chart illustrating number of endodontic treatment procedures done compared with dental implants in year 2000.
(Data from Millennium Research Group and ADA).
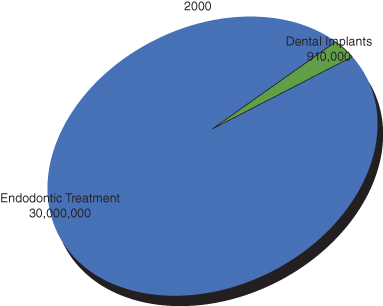
Figure 17.2 Pie chart illustrating the outcomes of 1,463,936 root canal–treated teeth with an 8-year follow-up.
Data are from Delta Dental insurance database and represent patients from all 50 states of the United States. (Data from Salehrabi and Rotstein 2004).
ENDODONTIC MICROSURGERY
Historically, surgical root canal treatment was approached with less enthusiasm by practitioners and educators due to lack of specialized surgical instruments, poor magnification, biologically incompatible materials, and low success rates. The introduction of the surgical operating microscope in endodontics in the 1990s allowed for the manufacturing of smaller, more convenient instruments, such as micro-mirrors, micro-condensers, and ultrasonics tips, which made the instrumentation and filling of the apical portion of the root canal system more precise and convenient to operators. The introduction of biocompatible materials resulted in superior, more predictable healing outcomes. The overall advancements in microsurgery resulted in better operators confidence, greater treatment success rate, and better patient experience.
Definition, Objectives, Indications, and Considerations of Endodontic Microsurgery
Definition and Objectives
Endodontic microsurgery can be best defined as a clinical procedure intended to remove the root tips, place a biocompatible material, and remove the associated diseased soft tissue. The optimal objectives—just like any endodontic procedure—are to prevent adverse signs and symptoms, to achieve complete removal of the contents of the root canal system(s), to create radiographically well-obturated root canal system(s), to promote healing and repair of the periradicular tissues, and to prevent further breakdown (AAE Quality Assurance Guidelines).
Indications
The indications for surgical root canal treatment include the following (Ingle, Craig, and Baumgartner 2007):
- Failure of nonsurgical root canal treatment or retreatment: If endodontic treatment or retreatment was provided within the standard of care and the patient had persistent signs and symptoms for longer than 4 years, this indicates failure of either procedure. Clinical symptoms of failure may require earlier intervention. While radiographic signs of failure can be followed up and diagnosed objectively, practitioners should look for other causes of failure such as missed canals, inadequate instrumentation and/or obturation, coronal leakage, and fractures. Often, retreatment of a contaminated, well-treated root canal system will result in resolution of signs or symptoms or is necessary before any surgical intervention.
- Failure of nonsurgical “initial” treatment and retreatment is not possible or practical or would not achieve a better result: The presence of a recent, serviceable crown or obstruction of the main root canal system by endogenous (e.g., calcification) or exogenous (e.g., post) reasons may render orthograde endodontic treatment impossible or impractical (Figure 17.3). It should be noted that if the initial endodontic treatment was done within the standard of care with no obvious reason for failure, practitioners should not attempt to retreat the tooth.
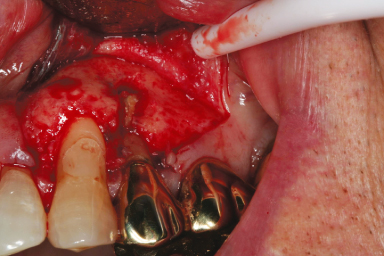
- A biopsy is necessary: If vertical root fracture is suspected, resecting the root apex followed by staining will often provide an accurate diagnosis of vertical root fracture (Figure 17.4). A biopsy for persistent infection (e.g., actinomycosis) can be done simultaneously with microsurgical root canal treatment.
Figure 17.3 (A, B) Indications for endodontic microsurgery: Mesial root canals obstructed at the coronal two-thirds due to extensive calcification; non-surgical treatment was impossible. Apical root resection was performed. Six-month follow-up radiograph.
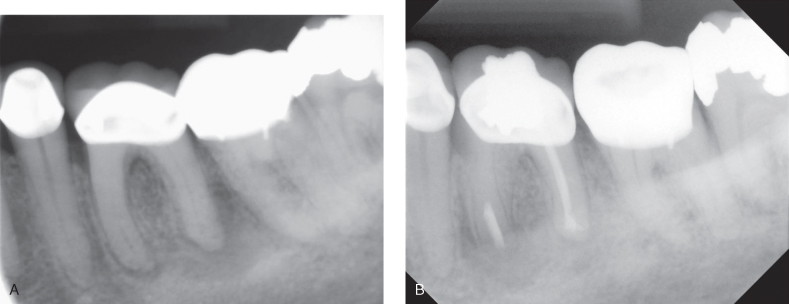
Figure 17.4 Tooth #12 had persistent periapical lesion determined radiographically after root canal treatment. Clinical examination during surgical root canal treatment showed vertical root fracture extending 6 mm from the root apex coronally.
Considerations
Almost any patient who is fit for oral surgical procedure is a good candidate for endodontic microsurgery, with few considerations:
- Patient medical status, including uncontrolled high blood pressure, recent myocardial infarction, subacute bacterial endocarditis, uncontrolled hematological problems, osteoradionecrosis, and uncontrolled diabetes
- Practitioner’s skills and experience and lack of equipment.
- Anatomical considerations, such as sulcus height, buccal thickness of bone, and proximity of mental foramen and inferior alveolar canal (Figure 17.5).
Figure 17.5 Anatomical considerations in endodontic microsurgery: Note the proximity of the mental foramen to the radiographic apex of tooth #20.
Other Considerations in Endodonitic Microsurgery
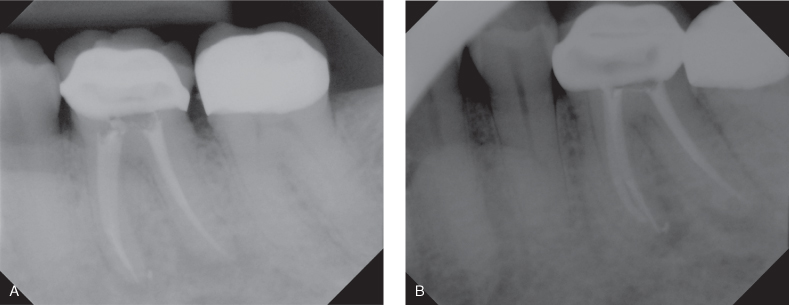
Before the decision for surgical root canal treatment to is made, several tooth-related factors should be evaluated. The restorability and the current crown/root ratio should be considered. It is generally accepted that 1:1 crown/root ratio is required for favorable radicular support for the prosthetic superstructure. Teeth with compromised crown/root ratio should be carefully evaluated since root-end resection will further compromise the root length. This is less important in multirooted teeth unless all roots will be resected (e.g., birooted premolars). It should be noted that surgical root canal treatment should be performed only if all other causes of endodontic failure are excluded. The current nonsurgical root canal treatment quality should be evaluated. Root canal–treated teeth should be assessed for radiographic obturation length, homogeneity, density, size, taper, and the presence of missed canals (Figure 17.6). Surgical treatment on a poorly done orthograde treatment does not satisfy the objective of endodontic treatment of complete removal of root canal content; retreatment should be chosen whenever possible as the first treatment approach over surgical treatment, provided that the benefits of this retreatment outweigh the surgical treatment (Moiseiwitsch and Trope 1998). Studies showed that success of surgical root canal treatment combined with orthograde treatment is higher than surgical root canal treatment alone (Dorn and Gartner 1990; Grung, Molven et al. 1990). Cases where coronal access is impossible due to recent crown restoration, presence of post(s), or other intracanal obstructions might render the retreatment difficult or impossible and should be assessed carefully since surgical treatment might be compromised by the previously done poor orthograde treatment. Adequate coronal restoration should be present since both a coronal and apical seal is required for successful endodontic treatment. Lack of adequate coronal seal results in coronal micro-leakage, which is an important cause of endodontic failure (Saunders and Saunders 1994). A retrospective study of 1,010 nonsurgical root canal treatments evaluating periradicular radiographic status found that failure was attributed more to technical quality of coronal restoration than to quality of root canal treatment itself (Ray and Trope 1995). If coronal leakage is suspected, the tooth first should be retreated nonsurgically and properly restored to ensure adequate coronal seal and followed up for evidence of healing.
Figure 17.6 (A) Straight shot of a tooth with apparently adequate root canal obturation. Patient failed to have the temporary restoration replaced by permanent for a long period. (B) Shift shot radiograph revealed additional cause for failure at distal root. Note the eccentric position of the distal root canal filling and dual lamina dura at distal root that suggests the presence of additional root canal systems. Retreatment of this case is the ideal treatment plan.
Surgical Procedure
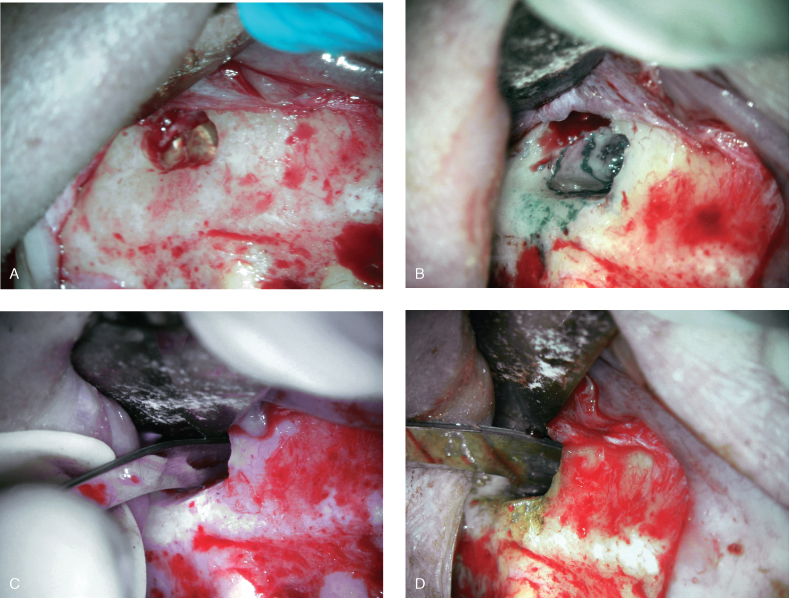
Endodontic microsurgery follows the same basic steps as any other surgical procedure that requires the elevation of full-thickness mucoperiosteal flap (Figure 17.7). The basic steps are as follows:
1. Obtain patient’s preoperative vital signs.
2. Review patient’s health history.
3. Deliver appropriate anesthesia.
4. Reflect full-thickness mucoperiosteal flap.
5. Perform osteotomy.
6. Perform curettage of lesion (if present) to obtain tissue for biopsy.
7. Complete root-end resection (apicectomy or apicoectomy) and root-end inspection.
8. Perform root-end preparation (retropreparation).
9. Complete root-end filling (retrofilling).
10. Perform flap reapposition and suture.
12. Give postoperative instructions and prescriptions and take vital signs.
13. Recall.
Figure 17.7 Specific steps of endodontic microsurgery: (A) osteotomy, (B) root-end resection, (C) retropreparation, (D) retrofilling.
Differences between Traditional and Modern Techniques in Endodontic Surgery
To be able to achieve the objectives of surgical root canal treatment, operators should be able to diagnose, access, and visualize the complex apical ramifications to ensure adequate cleaning and shaping and to be able to seal the remaining root canal system. Recent advances in endodontic microsurgery facilitated the diagnosis and management of failed initial endodontic treatments. Digital radiography introduced many potential benefits to endodontic practice with instant generation of high-quality images that allowed manipulation or processing of the captured image for enhanced diagnostic performance. Because patients needn’t be reexposed due to retakes and because there is lower dose exposure compared with D-speed film, there is a safer environment for patients. The associated ease of archiving and use for long-distance consultation, shorter turnaround times, reduction in time between exposure and image interpretation, and digital documentation of patient records made digital radiography a convenient alternative for practitioners and patients (Wenzel and Grondahl 1995; Naoum et al. 2003). Anesthetic techniques have improved, offering better patient comfort and hemostasis. Before the introduction of surgical operating microscope in 1990s, operators had to establish larger osteotomy sizes to be able to visualize root apices and to accommodate the larger instruments. Currently, osteotomy size is almost half the size and often dictated by the amount of root-end resection required. The introduction of micro-mirrors allowed better visualization of the resected root surface within the osteotomy. A steep bevel to allow better visualization and root-end preparation is no longer necessary, and operators are able to resect the root apex within 0–10 degrees. This conserves bone and root structure and reduces the amount of leakage due to exposure of dentinal tubules that result from steep bevels (Gilheany et al. 1994). The introduction of the surgical microscope allowed better inspection of the resected root surface to detect vertical root fractures, cracks, isthmuses, fins, and lateral canals. Commercially available ultrasonic instruments for microsurgeries became available in 1990s, and along with it came the introduction of superior handling and precise retropreparation within the root canal system compared to micro-hand-pieces that required bigger osteotomies and steep bevels. The use of amalgam is now considered below standard of care by most endodontists due to inferior biocompatability compared to the widely used mineral trioxide aggregate introduced in the 1990s. The introduction of finer monofilament suture materials resulted in superior soft tissue healing that allowed for early suture removal and superior patient experience. All those advancements resulted in bringing the overall success rate of endodontic microsurgery to a predictable range of 85%–96.8% compared to the wide-range success of 40%–90% before the microscope era.
Table 17.1. Differences between traditional and microsurgical endodontics.
Adapted from Kim and Kratchman (2006).
| Traditional | Microsurgery | |
| Time period | Before 1990s | Beginning of 1990s |
| Radiographs | Plain film | Digital |
| Anesthesia | Nerve block and infiltration | Nerve block, infiltration, intraosseous and intraligamentary |
| Microscope use | Never | Always |
| Osteotomy size | Approx. 8–10 mm | 3–4 mm |
| Bevel angle degree | 45–65 degrees | 0–10 degrees |
| Inspection of resected root surface | None | Always |
| Isthmus identification and treatment | Impossible | Always |
| Root-end preparation | Seldom inside canal | Always within canal |
| Root-end preparation instrument | Bur | Ultrasonic tips |
| Root-end filling material | Less biocompatible | More biocompatible |
| Sutures | 4–0 silk | 5–0, 6–0 monofilament |
| Suture removal | 7 days post-op | 2–3 days post-op |
| Healing success (over 1 year) | 40%–90% | 85%–96.8% |
Modern Advances in Microsurgical Instruments
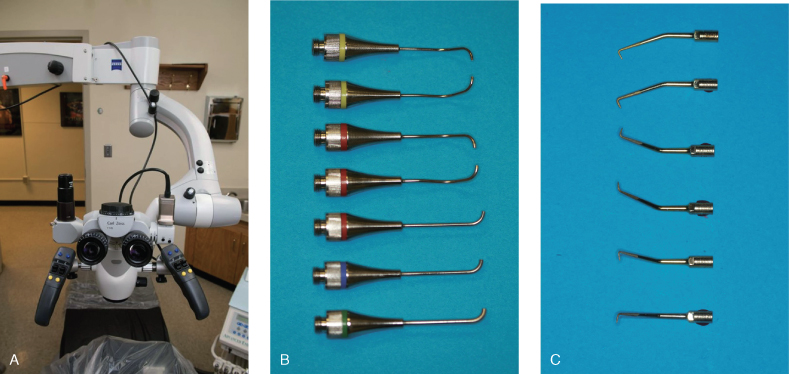
The introduction of the surgical operating microscope in endodontics allowed the development of smaller, more precise instruments (Figure 17.8). With higher magnification power up to 30× and better illumination, the need for bigger instruments and the removal of critical bone and tooth structure is greatly minimized. The osteotomy size is dictated by the size of the removed root apex instead of the instrument itself. The micro-mirrors, ultrasonic tips, micro-condensers, and micro-pluggers are almost the size of the osteotomy, if not smaller. Many instruments come with different angulations to accommodate difficult access in posterior teeth.
Figure 17.8 (A) Surgical operating microscope, (B) MAP system tips, (C) ultrasonic tips.
The Use of Ultrasonics in Endodontics
The use of burs in a micro-hand-piece for retropreparation had many drawbacks. The difficulty in access resulted in cavities that were never parallel to the main root canal system, do not confer to the overall shape of the canal, and often resulted in lingual perforation of the root. The difficulty in creating retropreparation of enough depth to retain the retrofilling was a common problem, and a steep bevel of resected root surface to improve visibility and extra means of retention in the retropreparation removed critical root structure. The introduction of ultrasonics in endodontics revolutionized surgical endodontics. Historically, the use of ultrasonics or ultrasonic instrumentation was first introduced to dentistry for cavity preparations using an abrasive slurry (Catuna 1953). The concept of using ultrasonics in endodontics was first introduced by Richman in 1957. However, it was not until Martin et al. (Martin 1976; Martin et al. 1980a, 1980b) demonstrated the ability of ultrasonically activated K-type files to cut dentin that this application found common use in the preparation of root canals before filling and obturation. The term “endosonics” was coined by Martin and Cunningham (1984, 1985) and was defined as the ultrasonic and synergistic system of root canal instrumentation and disinfection. The following is the most common use of ultrasonics in endodontics (Plotino et al. 2007):
- Access refinement, finding calcified canals, and removal of attached pulp stones.
- Removal of intracanal obstructions (separated instruments, root canal posts, silver points, and fractured metallic posts).
- Increased action of irrigating solutions.
- Ultrasonic condensation of gutta-percha.
- Placement of mineral trioxide aggregate (MTA).
- Surgical endodontics, including root-end cavity preparation and refinement and placement of root-end obturation material.
- Root canal preparation.
In the mid1980s, standardized instruments and aluminum oxide ceramic pins were introduced for retrograde filling. This system could not be used in cases with limited working space or in teeth with large oval canals (Keller 1990). Since sonically or ultrasonically driven microsurgical retro-tips became commercially available in the early 1990s (Pannkuk 1991), this new technique of retrograde root canal instrumentation has been established as an essential adjunct in periradicular surgery (Carr 1992). The use of ultrasonics in surgical endodontics overcomes many of the drawbacks that practitioners used to face when using burs. The small tips allow for better access and does not require large osteotomies to accommodate their size. Many tips come with different angulations for better access (Figure 17.9). The preparation is confined and parallel to the main root canal system and is of enough depth to retain the retrofilling material (Wuchenich et al. 1994). The reduced bevel results in conservative root resection, reduces the number of exposed dentinal tubules that result from steep bevels, and minimizes leakage (Tidmarsh and Arrowsmith 1989). Ultrasonics produced less smear layer in a retro-end cavity compared to a slow-speed hand-piece (Gorman et al. 1995). Several studies focused on crack formation as a result of root-end preparation. Gutmann and Saunders (1994) were first to report that ultrasonics may produce cracks during instrumentation. Abedi and Torabinejad reported that crack formation at the root end is a dependent on power setting, time, initial cracks, and thickness or remaining dentin (Abedi et al. 1995). Frank and Bakland found that the use of ultrasonics on medium power with water spray reduces incidence of root infractions (Ingle 2007). Other studies did not detect any crack formation associated with ultrasonic preparation (Beling et al. 1997; Waplington et al. 1997). The effect of microfractures on success has been studied by several authors. It should be noted that apical resorption after healing may eliminate the surface defects and contribute to the overall success of treatment (Holland et al. 1998). In conclusion, the superior results obtained with ultrasonics over burs resulted in higher overall success rates in surgical root canal treatment (Refer to surgical outcome studies Table 17.4).
Figure 17.9 Ultrasonics come in various angulations to facilitate access to the resected root surface.
The Advancements in Retrofilling Materials
The indications for the retrofilling procedure are to create a good seal and to prevent leakage of irritants from the root canal system (RCS) to the periradicular tissues (Bondra et al. 1989). This will improve the fill of complex root canal anatomy that would be missed or impossible to fill by orthograde obturation. A higher chance of failure is seen in teeth without an adequate apical seal (Frank et al. 1992). Placement of a retrofilling material is considered standard in endodontic microsurgery except in very few cases. The current evidence shows that today we have materials with better biologic and physical properties than those used in orthograde fillings. For example, mineral trioxide aggregate (MTA) has proven to provide better biologic and sealing ability and favors healing (Koh et al. 1997; Torabinejad et al. 1997). Significant numbers of practitioners use cold burnishing of the gutta-percha after root-end resection. Cold burnishing of gutta-percha exposed after apical root resection of a well-obturated canal resulted in a poorer apical seal than did no burnishing. Likewise, cold burnishing the gutta-percha exposed after apical root resection of a poorly obturated canal resulted in an improved apical seal compared with no burnishing (Minnich et al. 1989). The importance of root-end filling has been studied in literature. Table 17.2 shows the result of selected studies of the root apex management after root-end resection. Note that most of those studies have smaller sample size, used older techniques and materials as root-end fillings that do not reflect the current understanding, and resected root apexes to a level that would eliminate complex root-end anatomy, yet compromise the root structure. Although conclusive information is absent in the literature, retrofilling the conservatively resected root apex to provide an adequate seal should be done routinely, except in certain cases such as difficult access in second molars where visualization and instrumentation of the resected root apex is difficul/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


