Chapter 16
Surgical Endodontics: The Complimentary Approach
Richard Rubinstein and Alireza Aminlari
Department of Cariology, University of Michigan, Ann Arbor, MI, USA
Private Practice, Farmington Hills, MI, USA
Introduction
Treatment of apical periodontitis aims at eliminating the microbial contamination infection from the root canal system and preventing reinfection by sealing the root canal space. However, even when the most careful clinical procedures are followed, a proportion of lesions may persist because of the anatomical complexity of the root canal system, which are inaccessible to mechanical and chemical debridement (1), as well as microorganisms capable of surviving and proliferating in apical tissues, thus enabling a persistent infection interfering with posttreatment healing of the lesion (2–5). Ricucci and Siqueira demonstrated that when pulp necrosis reached the apical third, all lateral canals and apical ramifications contained either partially or completely necrotic tissue (6). Chemomechanical preparation partially removed necrotic tissue from the entrance of the lateral canals and apical ramifications, whereas the adjacent tissue remained inflamed, sometimes infected, and associated with apical disease.
The debate whether chronic apical lesions are infected is still a subject of controversy. Kronfeld stated bacteria were always found within the root canal while apical tissue is not an area in which bacteria live but are destroyed (7). In contrast, Stewart, in 1947, proposed that bacteria were present in apical lesions (8). Few years later, Hedman demonstrated that 68% of 82 apical lesions contained bacteria (9). Tronstad et al. demonstrated that bacteria can survive in apical lesions and proved endodontic infections (4, 10). Advanced molecular biology and polymerase chain reaction (PCR) permitted identification of more species of microbes capable of inhabiting apical lesions and providing strong evidence that extraradicular infection might be the cause of many failed endodontic treatment (11). In 2000, Sunde et al. examined whether bacteria were present in apical lesions of asymptomatic teeth before sampling or were transferred there during sampling (3). The predominant cultivable bacteria were anaerobic and clearly different from the bacteria present at the neighboring sites and appeared to have been there before sampling. Siqueira and Ricucci found actinomycosis and arachnea in apical lesions (5, 12). Although no stainable bacteria were observed in the apparently well-treated main canal, apical ramifications were clogged with dense bacterial biofilms that were contiguous to extraradicular actinomycotic aggregates (5). Current literature supports the fact that extraradicular biofilms are related to refractory apical periodontitis, mostly by aggregation of numerous filamentous or spirochete-shaped bacterial cells (13, 14) (Figure 16.1). Clinically this implies that even if the tooth is extracted (ultimate root canal), apical periodontitis might persevere and develop a residual cyst (Figure 16.2). Thus the etiology is outside the root canal system and surgical intervention (along with orthograde treatment) is necessary to achieve apical healing.
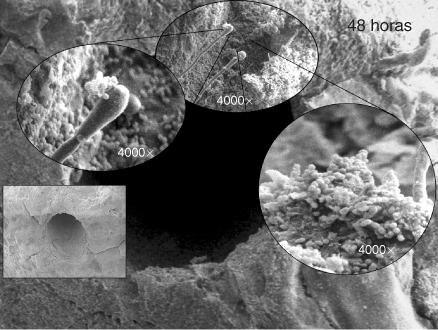
Figure 16.1 High power showing the apical foramen and cementum covered by a biofilm. Another area of the external surface of the apical surface covered by a biofilm. Note interaction and synergy between Enterococcus faecalis and Candida.
(Image courtesy of Dr. Ana Maria Gonzalez.)
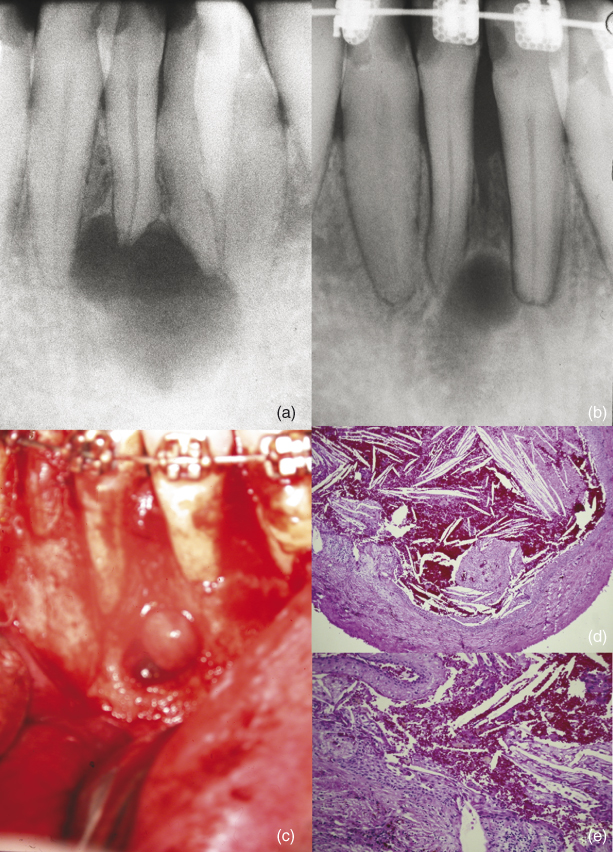
Figure 16.2 (a) Clinical radiograph of a lower lateral incisor with an apical lesion. The tooth was extracted for orthodontic reasons. The apical area was not curetted. (b) Postextraction radiograph with orthodontic treatment in progress. (c) Surgical removal of the persistent lesion. (d) Biopsy showing an epithelial lining cholesterol clefts and chronic inflammation consistent with the diagnosis of a residual cyst. (e) Higher power of the lesion showing epithelium, cholesterol clefts, and inflammation in the connective tissue.
Although the pathogenesis of apical periodontitis has been well described (15–17), it must be emphasized that the composition of lesions of apical periodontitis at any time depends on the balance between the microbial factors and the host defenses. Consequently, a great deal of morphological heterogeneity is common for chronic apical periodontitis.
Extraradicular endodontic plaque
In 1987, Tronstad et al. (10) demonstrated the presence of extraradicular endodontic plaque on the external surface of necrotic teeth. In a clinical and histopathologic study, Ricucci and Siqueira observed the presence of extraradicular biofilms in 6% of the cases (18). This has been substantiated with the visualization of biofilms under Scanning Electron Microscopy (SEM) (Figure 16.3).
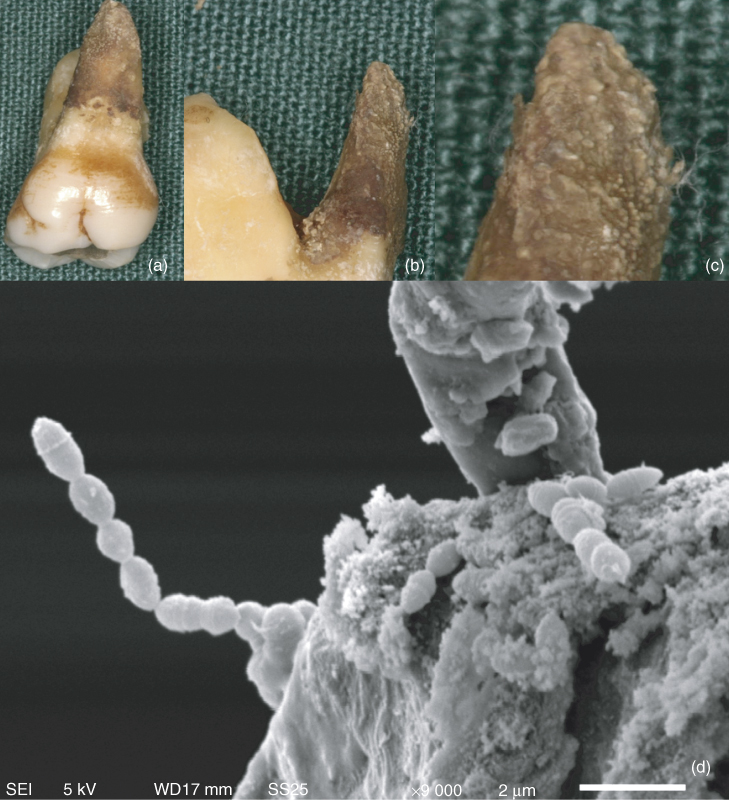
Figure 16.3 (a–c) Extracted maxillary molar demonstrating the presence of biofilm covering the external surface of the palatal root. (d) Scanning electron micrograph of the extraradicular biofilm at high power (×9000) showing the diversity of bacteria.
Bacteria and apical actinomycosis
Actinomycosis is a chronic, granulomatous, infectious disease in man and animals caused by the genera Actinomyces and Propionibacterium (which normally colonize the mouth, colon, and vagina). Invasion of the microorganism is usually a result of the disruption of the mucosal barrier after trauma or dental manipulation (1). The endodontic infections of actinomyces are a sequel to caries and are caused by Actinomyces israelii and Propionibacterium propionicum, commensals of the oral cavity. Because of the ability of the actinomycotic organisms to establish in apical tissues, they can perpetuate the inflammation, even after orthograde root canal therapy. Among the most commonly isolated are A. israelii and P. propionicum, which are associated with refractory lesion (19). The properties that enable these bacteria to establish in the apical tissues are not fully understood, but appear to involve their ability to build cohesive colonies that enable them to escape the host defense system. There are only limited data on the frequency of apical actinomycosis among apical lesions or on the correlation between apical and cervicofacial actinomycosis (19).
Virus
Within the past decade, researchers have reported the presence of certain viruses in inflamed apical tissues and suggested as a possible etiological pathogenic factor related to apical periodontitis. Both Cytomegalovirus and Epstein–Barr viruses are present in almost all humans in latent form from previous primary infections (1). Sabeti et al. (20) investigated the occurrence of herpes viruses in apical granulomas. cDNA identification of genes transcribed late during the infectious cycle of herpes viruses was used to indicate an active herpes virus infection. The authors proposed that herpes viruses may cause apical pathosis as a direct result of virus infection and replication or as a result of virally induced damage to the host defense (21). In addition, a strong association of human Cytomegalovirus and Epstein–Barr virus with the acute exacerbation of apical lesions has been reported (20, 22). Apical lesions harboring dual Cytomegalovirus and Epstein–Barr virus infection tended to exhibit elevated occurrence of anaerobic bacteria, be symptomatic, and show large-size radiographic bone destruction. Cytomegalovirus and Epstein–Barr virus, in cooperation with specific bacterial species, have also been associated with various types of advanced marginal odontitis and several nonoral infectious diseases (22). Slots et al. have postulated that virus may initiate lesions by attacking and inactivating dendritic cells, thus processing and presenting the antigen by the dendritic cells to the B and T cells (21). This weakens the immune and inflammatory response allowing bacteria to follow the viruses into the PA lesion.
Fungi
Fungi have also isolated in apical lesions but in far rear instances. They are thought to be the secondary contaminants following bacteria through the apical foramen. Among fungi, Candida is the most frequently found, although other fungi have been cultivated. It has been detected in therapy-resistant apical lesions (23) (Figure 16.4).
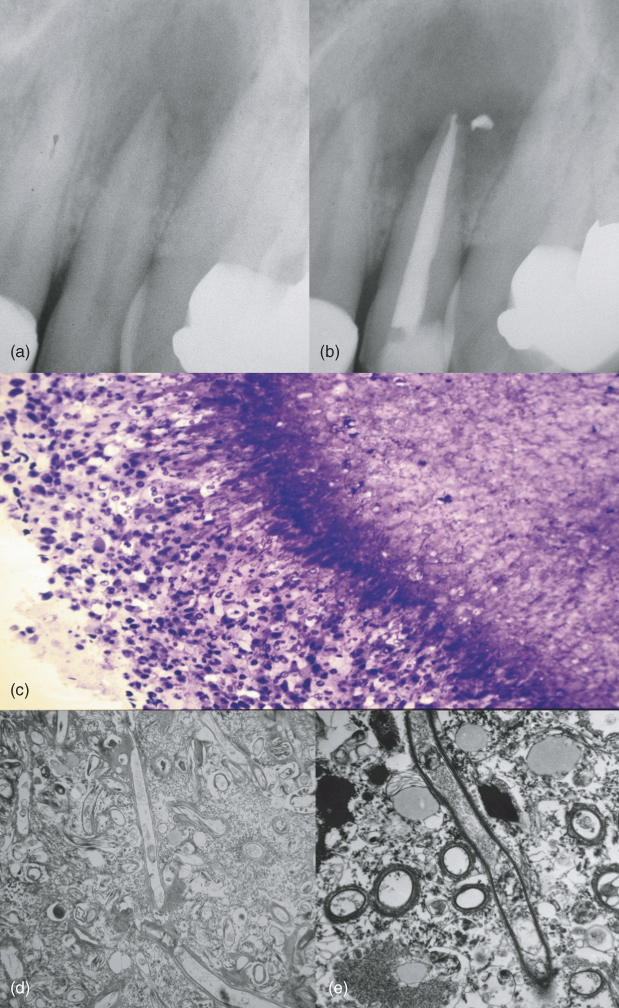
Figure 16.4 (a) Preoperative radiograph of a maxillary lateral incisor with an apical radiolucency. (b) Three-month recall demonstrating an increase in the size of the lesion. (c) One micron section prior to electron microscopy and stained with methylene blue. The inflammatory cells are attacking the antigen. (d) Low power electron micrograph showing fungal hyphae. (e) Higher power showing the presence of the fungus.
Cholesterol
As a result of the inflammatory process with bacterial and cellular destruction, cholesterol is a usual component of apical lesions. Nair has stated that the body cannot destroy cholesterol and thus is a reason for the nonhealing of nonsurgical root canal treatment (24). It can span from only several cholesterol clefts to the predominant component of the lesion. Thoma and Goldman classified these lesions as cholesteatoma where cholesterol is the dominating feature of the lesion (25). These lesions do not appear to heal with orthograde treatment and must be surgically removed for healing to occur (Figure 16.5).
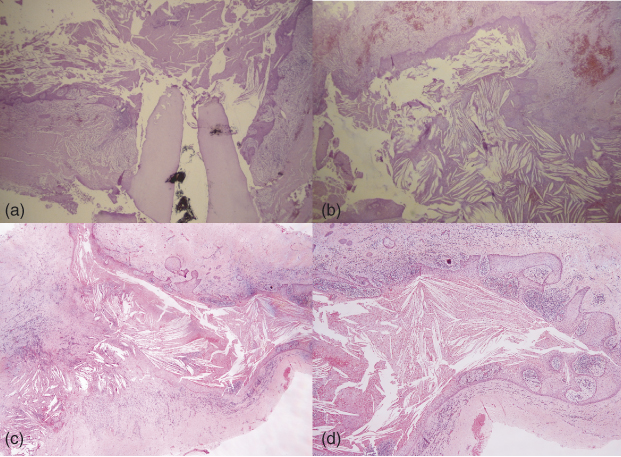
Figure 16.5 (a) Biopsy of a large lesion with the previously treated root tip. Note the cyst’s lumen filled with cholesterol clefts. (b) Another part of the same lesion showing an epithelial lining and cholesterol clefts. (c) Epithelium and cholesterol clefts consistent with a cystic cholesteatoma. (d) Higher power of the same lesion.
Foreign bodies
Theoretically, anything that is in the mouth is capable of penetrating into the canal system and if small enough into the apical area. Numerous reports of foreign bodies and materials that penetrate the apical foramen along with bacteria show a foreign body giant cell reaction. Along with the usual acute and chronic inflammatory response, the macrophages coalesce to form giant cells in an attempt to degrade the foreign material. These lesions must be curetted in order to effect healing (Figure 16.6).
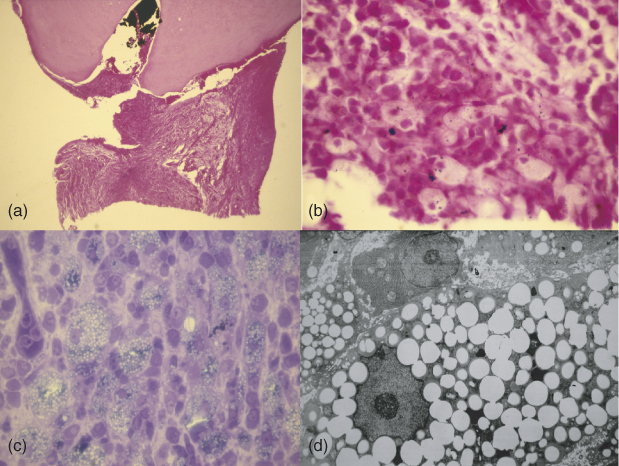
Figure 16.6 (a) Histologic section of an extracted tooth with attached lesion. Note foreign material in the canal. The bottom part of the lesion was used for the transmission electron microscope. (b) High power of the lesion showing macrophages and foreign material. (c) One micron section stained with methylene blue showing the macrophages full of foreign material. (d) A macrophage full to bursting with lipid. Note the absence of organelles and impingement on the nucleus.
Anatomical complexities
Walter Hess (26), a Swiss dentist, first published his landmark anatomical studies in the early 1920s. When his work was first published many clinicians felt that the anatomical complexities he reported were artifacts created by injecting vulcanite rubber under too much pressure (Figures 16.7 and 16.8). However, more progressive thinkers of that time believed the results had merit and sought more effective ways to clean, shape, and obturate root canal systems. More recently, Takahashi and Kishi (27), using a dye infusion process, also studied anatomical complexities. These models clearly show the majesty and grace of the human dental pulp (Figures 16.9–16.12). Weller et al. (28) studied the incidence and location of the isthmus in the mesial buccal root of the maxillary first molar and found a partial or complete isthmus 100% of the time at the 4 mm level of resection. West (29) looked at the relationship between failed endodontics and unfilled or underfilled portals of exit (POE’s). Using a centrifuged dye, he identified that 100% of the failed specimens studied had at least one underfilled or unfilled POE. As 93% of the canal ramifications occur in the apical 3 mm (27), it is logical that the clinicians attempt to treat the root canal system to the full extent of the anatomy. Failure to address these anatomical concerns will leave the etiology of failure unattended and reinfection, even after the removal of a apical lesion, may occur. Clearly, root canal systems are more complex than previously thought. Significant pulpal anatomy such as accessory canals and isthmuses has to be considered when performing both conventional endodontic treatment and apical surgery. The acceptance of the significance of these anatomic complexities and the need to disinfect them may in fact have been the genesis of modern apical surgery, which could further be appreciated with the introduction of magnification and microsurgical armamentarium.
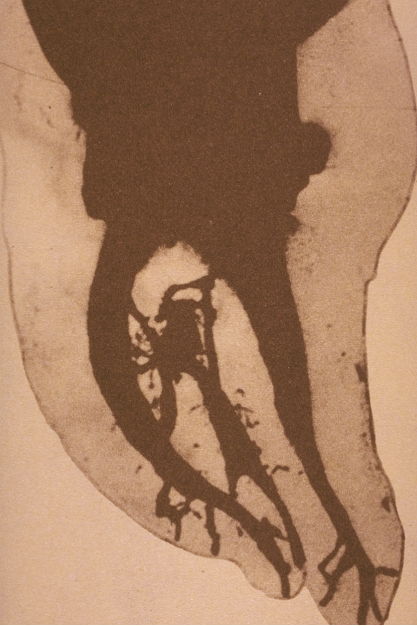
Figure 16.7 Hess model of a mandibular molar showing anatomical complexities throughout the root canal system.
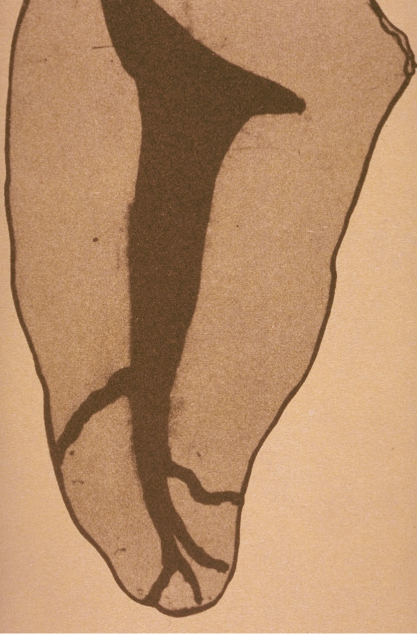
Figure 16.8 Hess model of a mandibular bicuspid showing anatomical complexities in the apical terminus.
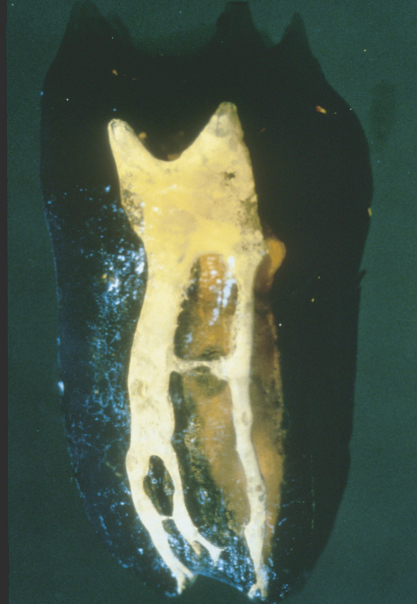
Figure 16.9 Takahashi model of the mesial view of the mesial root of a mandibular molar. Note the mid-root isthmus and the apical bifidity of the buccal canal. Also note the multiple apical termini.

Figure 16.10 Takahashi model of a mandibular second bicuspid. Note how the single canal bifurcates, rejoins, and then splits once more at the canal terminus.
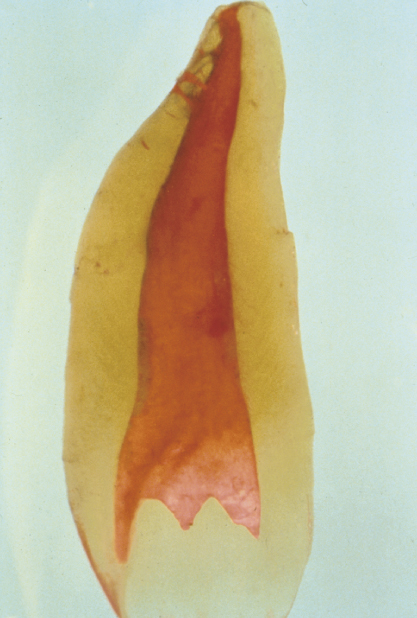
Figure 16.11 Takahashi model of a maxillary central incisor. Note the multiple portals of exit in the apical third of the root.
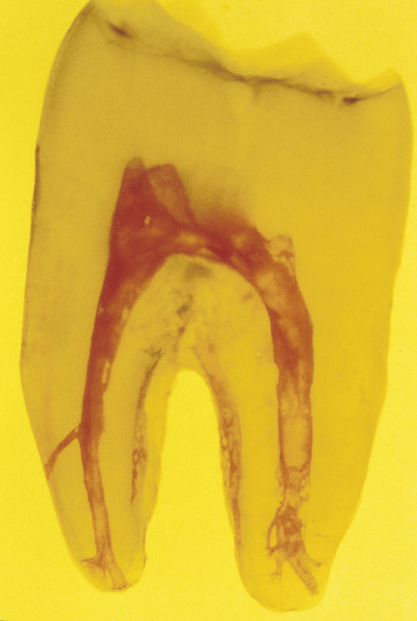
Figure 16.12 Takahashi model of a mandibular molar. Note the anatomical complexities present in both roots.
Apical microsurgery
In order to understand the objectives of apical microsurgery, one can divide the procedure into multiple stages or sections. Among these are flap design, flap reflection, flap retraction, osteotomy, apical curettage, biopsy, hemostasis, apical resection, resected apex evaluation, apical preparation, apical preparation evaluation, drying the apical preparation, selecting retrofilling materials, mixing retrofilling materials, placing retrofilling materials, condensing retrofilling materials, carving retrofilling materials, finishing retrofilling materials, and flap closure.
After anesthesia is obtained, and prior to incising the surgical flap, the oral cavity should be rinsed with a disinfectant solution such as chlorhexidine. A 0.12% chlorhexidine rinse has been shown to significantly reduce the bacterial count in the oral cavity in advance of operative procedures (30).
Microscalpels (Figure 16.13) (SybronEndo, Orange, CA) are used in the design of the flap to delicately incise the interdental papillae when full-thickness flaps are required. Microscalpels cause less trauma than conventional scalpels resulting in less scarring and more favorable cosmetic outcomes. Vertical incisions are made 1.5–2 times longer than in conventional apical surgery to assure that the flap can easily be reflected out of the light path of the microscope.
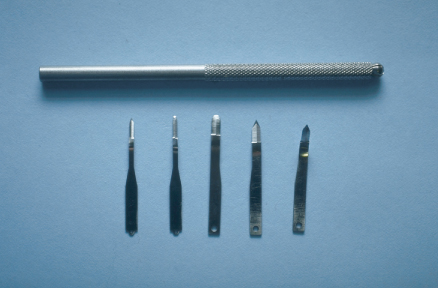
Figure 16.13 A variety of microscalpels sized 1–5 used for precise incision.
Historically, flaps have been reflected with a Molt 2–4 curette or variation of the Molt 2–4. This instrument is double ended and the cross-sectional diameters of the working ends are 3.5 and 7 mm. Under low-range magnification it can readily be seen that even the smallest end of this instrument is too large to place beneath the interdental papilla without causing significant tearing and trauma to the delicate tissues. Rubinstein Mini-Molts (Figure 16.14) (JEDMED, St. Louis, MO) are available in two configurations whose working ends are 2 and 3.5 and 2 and 7 mm. The smaller ends of these instruments provide for atraumatic elevation of the interdental papilla making flap reflection more predictable and gentle to the tissues. The recently introduced PR-1 and PR-2 (Figure 16.15) (G. Hartzell & Son, Concord, CA) have similar geometries and also accomplish the goals of atraumatic flap reflection.

Figure 16.14 A comparison of the small ends of two mini-Molts and a standard Molt 2–4 curette.
Figure 16.15 PR-1 and PR-2 periosteal elevators.
Once the flap has been reflected, instruments such as the Minnesota retractor have been used to retract the flap away from the surgical field while assuring visual access. Maintaining pressure on this instrument for even a short period of time often causes restriction of blood flow to the fingers of the operator and using it can be quite uncomfortable. A series of six retractors (JEDMED, St. Louis, MO) (Figure 16.16) and a new universal positioning retractor offering a variety of serrated contact surfaces that are flat, notched, and recessed allow the operator several options for secure placement in areas of anatomical concern. Among these are placements over nasal spine, canine eminence, and mental nerve. These retractors decrease the chance of slippage, which can cause trauma to the flap and delicate gingival mucosa. The blades of the retractors are designed to retract both the flap and the lip and are bent at 110° to keep the retractor and operator’s hand out of the light path of the microscope. The handles are ergonomically designed to decrease cramping and fatigue and can be held in a variety of grips.

Figure 16.16 Blade and contact surfaces of the Rubinstein retractors 1–6.
Because we can see better with the surgical operating microscope (SOM), bone removal can be more conservative. Handpieces such as the Impact Air 45™ (SybronEndo, Orange, CA) introduced by oral surgeons to facilitate sectioning mandibular third molars, are also suggested for apical surgery to gain better access to the apices of maxillary and mandibular molars. When using the handpiece, the water spray is aimed directly into the surgical field but the air stream is ejected out through the back of the handpiece, thus eliminating much of the splatter that occurs with conventional high-speed handpieces. Because there is no pressurized air or water, the chances of producing pyemia and emphysema are significantly reduced.
Burs such as Lindemann bone cutters (Brasseler USA, Savannah. GA) are extremely efficient and are recommended for hard tissue removal. They are 9 mm in length and have only four flutes, which result in less clogging. With the use of an SOM, the Impact Air 45 and high-speed surgical burs can be placed even in areas of anatomical jeopardy with a high degree of confidence and accuracy (Figure 16.17). The size of the osteotomy should be as small as practical so that healing will not be impaired, yet large enough to allow for complete debridement of the bony crypt and access for root-end procedures that will follow.
Figure 16.17 Impact Air 45™ and surgical length bur in close proximity to the mental nerve 8×.
With the SOM, apical curettage is facilitated because bony margins can be scrutinized for completeness of tissue removal. A Columbia 13–14 curette is recommended in small crypts because it is curved and can reach to the lingual aspect of a root. After the Columbia 13–14 is used, the Jacquette 34/35 scaler is recommended to remove the remainder of granulomatous tissue. Because of its sharp edge, the Jacquette 34/35 is an excellent instrument for removing granulomatous tissue from the junction of the cemental root surface and the bony crypt. The more tissue that can be removed results in less work for the body to do relative to wound healing. In addition, any foreign material present in the bony crypt should be removed as it could cause persistent irritation and may prevent complete healing of the tissues (31). After the bony crypt has been physically debrided, it should be rinsed thoroughly with sterile saline.
There is general agreement that the main cause of failure in conventional endodontic treatment is the clinician’s inability to adequately shape, disinfect, and obturate the entire root canal system (32). As previously stated (27–29), the majority of this untreated anatomy is located in the apical 3 mm and for this reason a 3 mm resection is recommended. With the introduction of ultrasonics for creating root-end preparations, a second reason for a 3 mm resection has emerged. Layton et al. (33), Beling et al. (34), Min et al. (35), Morgan and Marshall (36) and Rainwater et al. (37) have studied the incidence of craze line, cracks, and fractures in the root and cemental surfa/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


