CHAPTER 16. Oral premalignancy
Various oral mucosal lesions, particularly red lesions (erythroplasias) and some white lesions (leukoplakias), have a potential for malignant change (Table 16.1). In some of them, such as chronic hyperplastic candidosis, the risk may be very low. By contrast, the risk with erythroplasia is exceedingly high.
| *Risks of malignant change are difficult to determine accurately and vary with many factors discussed below. If more than 25% of lesions become malignant in 5 years this is considered an exceptionally high risk. Malignant change in 2–3% of lesions in 5 years is considered a relatively low risk. Note that the highest risk lesions are uncommon. Common lesions appear in between 1 and 5% of the population. |
|||
| Lesion/condition | Aetiology | Risk of malignant change* | Prevalence in the UK |
|---|---|---|---|
| Dysplastic leukoplakia | Unknown | High, but can regress | Uncommon |
| Erythroplasia | Idiopathic/smoking | Very high | Rare |
| Speckled leukoplakia | Idiopathic/smoking | High | Rare |
| Tertiary syphilis | Treponema pallidum | Very high | No longer seen |
| Oral submucous fibrosis | Betel quid chewing | High | Uncommon |
| Dyskeratosis congenita | Genetic | High | Rare |
| Pipe smoker’s keratosis | Pipe smoking | Low and not in the keratotic area | Becoming uncommon |
| Snuff-dippers’ keratosis | Smokeless tobacco | Low | Uncommon |
| Chronic candidosis | Candida albicans | Low | Uncommon |
| Lichen planus | Idiopathic | Low | Common |
| Discoid lupus erythematosus | Autoimmune | Unclear (mainly lip) | Uncommon |
In general, the most common white lesions have the lowest risk of malignant transformation. Practitioners will see many oral white lesions, but few carcinomas. However, they must be able to recognise lesions at particular risk and several features help to assess the likelihood of malignant transformation. The accuracy of such predictions is low, but the process of identifying ‘at risk’ lesions is fundamental to diagnosis and treatment planning. Important factors are listed in Box 16.1 and information on each of these should be sought.
Box 16.1
Risk factors for malignant change in white lesions
History
Clinical aspects
• Advanced age
• Areas of reddening in the lesion
• Areas of speckling in the lesion
• Nodular or verrucous areas or ulceration
• High-risk site:
posterolateral tongue
floor of mouth
retromolar region
anterior pillar of fauces
• Large lesions
• Lesions present for long periods
• Enlargement or change in character of pre-existing lesion
Special investigations
• Degree of dysplasia on biopsy
The best predictor of the potential for malignant transformation is the degree of dysplasia seen histologically. For this reason, and because a few lesions will already be malignant, biopsy of red and white patches is mandatory. The term dysplasia (literally, abnormal growth) is given to the cytological abnormalities seen in both malignant and premalignant cells (Box 16.2). Premalignancy is distinguished from malignancy only by the latter’s invasiveness and release of metastases.
Box 16.2
Epithelial dysplasia: histological features
• Drop-shaped rete ridges
• Nuclear hyperchromatism
• Nuclear pleomorphism and raised nuclear/cytoplasmic ratio
• Excess mitotic activity
• Loss of polarity of cells
• Deep cell keratinisation
• Disordered or loss of differentiation
• Loss of intercellular adherence
Nuclei stain more densely due to greater nucleic acid content. They are variable in size, out of proportion to that of the cell, so that there may be little surrounding cytoplasm. Mitoses may be frequent and at superficial levels. Loss of polarity refers to the fact that basal cells in particular may lie higgledy-piggledy at angles to one another. Deep cell keratinisation (dyskeratosis) refers to individual cells which start to keratinise before the surface is reached and show eosinophilic change deeply within the epithelium. As differentiation is lost, organisation of the individual cell layers deteriorates and clearly-defined basal and spinous cell layers cannot be identified. Drop-shaped rete ridges are regarded as a particularly adverse feature. With loss of intercellular adherence, the cells become separated.
A lymphoplasmacytic infiltrate of highly variable intensity is usually present in the corium.
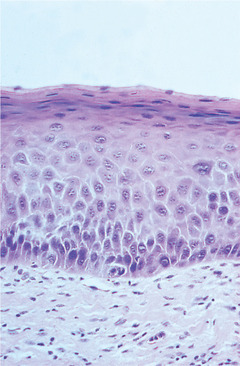
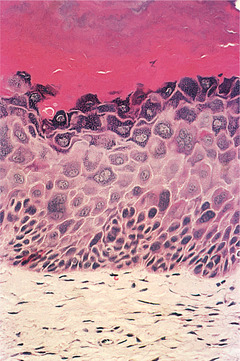
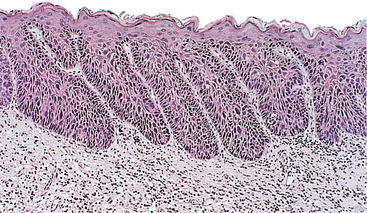
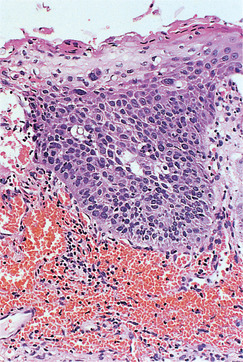
Dysplasia is usually graded as mild, moderate and severe as a guide to patient management (Fig. 16.1, Fig. 16.2, Fig. 16.3 and Fig. 16.4). Carcinoma-in-situ is a term sometimes used for the most severe dysplasia where the abnormalities extend throughout the thickness of the epithelium, a state sometimes graphically called ‘top-to-bottom change’. In such a lesion, all the cellular abnormalities characteristic of malignancy may be present; only invasion is absent. The significance of this grading is discussed below, but the histological assessment of oral epithelial dysplasia is notoriously unreliable because it is subjective and changes are not reliably correlated with behaviour.
 |
| Fig. 16.1
Mild dysplasia. In this lesion there is a thin layer of parakeratin and the structure, maturation and orderly differentiation of the epithelial cells is largely unaffected. However, there is a degree of irregularity of basal cells with variation in size and hyperchromatism.
|
 |
| Fig. 16.2
Mild dysplasia. In this lesion there is prominent orthokeratosis and a keratohyaline layer immediately below it. Dysplasia is more prominent than in the previous figure, with enlarged hyperchromatic and bizarre cells in the basal and lower prickle cell layers.
|
 |
| Fig. 16.3
Moderate dysplasia. The dermal papillae extend close to the surface and there are elongated rete processes, some of which are broader deeply. Enlarged and hyperchromatic cells are visible at this low power in rete processes and in most of the prickle cell layer.
|
 |
| Fig. 16.4
Severe dysplasia. This rete process is composed almost entirely of cells with dark and irregularly shaped nuclei. Only the most superficial layers of cells show maturation to squamous cells and the orderly maturation and differentiation of epithelial cells has been lost.
|
PREMALIGNANT LESIONS AND CONDITIONS
Premalignant lesions are those lesions in which carcinoma may develop. Premalignant conditions are associated with a risk of carcinoma at some site within the mouth, not necessarily marked by a pre-existing lesion.
ERYTHROPLASIA (‘ERYTHROPLAKIA’) → Summaries pp. 277, 279
Erythroplasias are red patches. The surface is frequently velvety in texture and the margin may be sharply defined. Lesions of this type typically do not form plaques (hence the term ‘erythroplakia’ is misleading) but, instead, they are flat or depressed below the level of the surrounding mucosa (Fig. 16.5). Erythroplasia is uncommon in the mouth but carries the highest risk of malignant transformation and almost half of lesions turn out to be malignant on first biopsy.
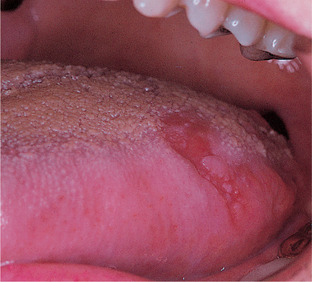 |
| Fig. 16.5
Erythroplasia. This slightly depressed, well-defined red patch on the dorsolateral tongue showed squamous carcinoma on biopsy.
|
Pathology
Erythroplastic lesions usually show epithelial dysplasia which may be severe. In other cases, there may be micro- or frankly invasive carcinoma. The epithelium is atrophic and this, together with inflammation, accounts for the red colour seen clinically.
SPECKLED LEUKOPLAKIA → Summary p. 277
This term applies to lesions consisting of white flecks or fine nodules on an atrophic erythematous base (Figs 16.6 and 16.7). They can be regarded as a combination of or transition between leukoplakia and erythroplasia. Speckled leukoplakia also more frequently shows dysplasia than lesions with a homogeneous white surface. The histological characteristics are usually, therefore, intermediate between leukoplakia and erythroplasia.
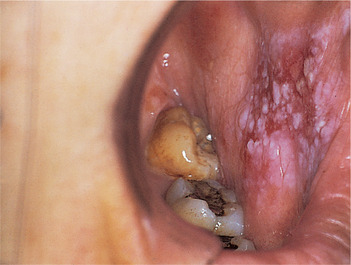 |
| Fig. 16.6
Speckled leukoplakia. A poorly-defined speckled leukoplakia on the cheek of an elderly female. Carcinoma was present at the first biopsy.
|
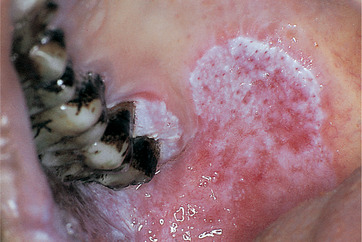 |
| Fig. 16.7
Premalignant lesion in a betel quid chewer. The classical appearance of a speckled leukoplakia such as this is almost always associated with either severe dysplasia or invasive carcinoma. Note also the brown betel quid staining on the teeth.
|
Many cases of chronic candidosis have this appearance.
IDIOPATHIC LEUKOPLAKIA → Summaries pp. 277, 278
Leukoplakia is defined as a white patch which cannot be wiped off the mucosa or ascribed to any specific disease process. Although the term is often used loosely for any white patch, it should be reserved for idiopathic lesions. If investigations fail to reveal any cause, the term may be appropriate.
The most extensive follow-up studies on white patches suggest that idiopathic leukoplakia now has the highest risk of developing cancer, especially now that late-stage syphilis forms a negligibly small group. Nevertheless, the malignant transformation rate of leukoplakia is relatively low, around 1–2% in 5 years and, even in smokers, the vast majority of leukoplakias show no dysplasia histologically and carry no risk of malignant transformation.
Clinical features
Idiopathic leukoplakias and dysplastic lesions do not have any specific clinical appearance, but are tough and adherent and typically form plaques whose surface is slightly raised above the surrounding mucosa. The surface is usually irregular. Small and innocent-looking white patches are as likely to show epithelial dysplasia as large and irregular ones (Figs 16.8 and 16.9). However, lesions with red, nodular or verrucous areas (Fig. 16.10) should be regarded with particular suspicion. The most common sites are the posterior buccal mucosa, retromolar region, floor of mouth and tongue.
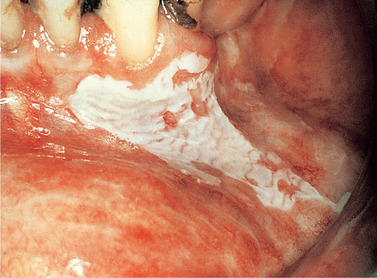 |
| Fig. 16.8
Homogeneous leukoplakia. There is a bright, white, sharply-defined patch extending from the gingiva on to the labial mucosa. The surface has a slightly rippled appearance and no red areas are associated.
|
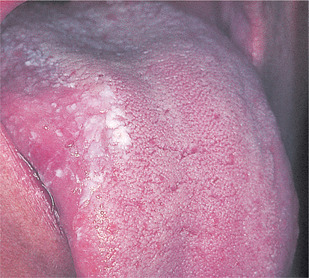 |
| Fig. 16.9
An innocent-looking, poorly-defined inconspicuous white patch which showed dysplasia on biopsy. Despite excision, malignant transformation followed several months later.
|
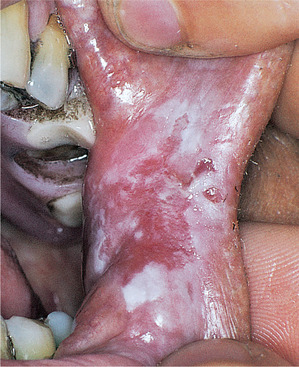 |
| Fig. 16.10
White patch with dysplasia. This postcommissural lesion is poorly defined and comprises both red and white areas. Lesions at this site are frequently due to candidosis.
|
Pathology
The histopathology is highly variable, but there is always parakeratosis or orthokeratosis, or both in different areas, and the two may alternate along the length of the specimen. The keratin gives the lesion its white appearance. The epithelium ranges from thinner than normal (atrophic) to much thicker (acanthotic) (see Figs 16.1 and 16.2).
Most leukoplakias show no dysplasia histologically. A minority display a range of dysplasia from mild to severe and treatment is planned partly on this basis. An inflammatory reaction of lymphocytes and plasma cells induced by the abnormal dysplastic cells is often present in the underlying connective tissue.
SUBLINGUAL KERATOSIS
The term ‘sublingual keratosis’ is applied to white lesions on the floor of mouth and ventral tongue. Whether this lesion is a different entity from other leukoplakias is unclear. Malignant change was reported in an unusually high proportion of cases (30%) in one series, but this has not been widely confirmed. Probably the risk of malignant transformation is less than 10% and possibly much lower.
Clinical features
Sublingual keratosis is a white, soft plaque, usually with a finely wrinkled surface, an irregular but well-defined outline and sometimes bilateral with a butterfly shape. The plaque typically extends from the anterior floor of the mouth to the undersurface of the tongue (Figs 16.11 and 16.12). There is usually no associated inflammation.
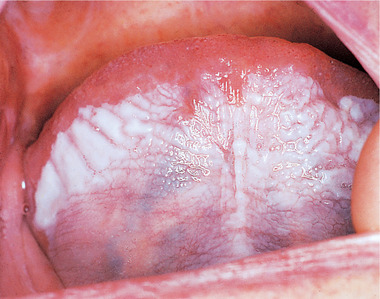 |
| Fig. 16.11
Sublingual keratosis. This white patch involving the entire ventral tongue and floor of mouth has a uniformly wrinkled appearance. No red areas are associated but the site alone may possibly indicate a high risk of malignant transformation.
|
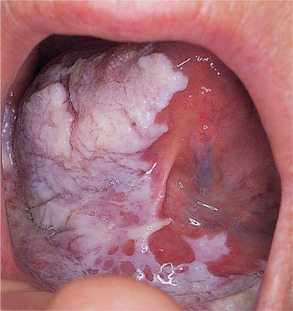 |
| Fig. 16.12
Sublingual keratosis. This more irregular white patch is associated with some reddening in the floor of the mouth.
|
Pathology
Sublingual keratosis is not distinctive histologically and the appearances are those described above for leukoplakia.
PROLIFERATIVE VERRUCOUS LEUKOPLAKIA
This is a rare and rather poorly defined clinical presentation of multiple white lesions with a very high risk of transformation. Lack of specific features makes it impossible to make the diagnosis confidently until many years have passed or carcinoma develops. In order for the diagnosis to have any value, it is important that the criteria are strictly applied. Not all verrucous leukoplakias are proliferative verrucous leukoplakia.
Clinical features
Patients are elderly and mostly female. Many are non-smokers. They develop flat leukoplakias that, over a period of decades, develop a verrucous surface and progress inexorably to verrucous or squamous carcinoma. Common sites affected are buccal mucosa, gingiva and tongue. It is common for several separate primary carcinomas to develop over many years. Lesions are difficult or impossible to eradicate surgically and recur or spread to new sites.
Pathology
The histological features of the lesions are those of idiopathic leukoplakia, with or without dysplasia.
PIPE SMOKER’S KERATOSIS → Summary p. 277
As noted earlier (Ch. 15), palatal keratosis due to pipe-smoking is benign. Any carcinomas related to pipe-smoking appear in another site in the mouth and may not be preceded by keratosis.
SMOKELESS TOBACCO-INDUCED KERATOSES
The majority of tobacco used worldwide is dried, cured and smoked in cigarettes or cigars. However, many cultures have traditional tobacco habits that use snuff or crude tobacco applied topically to the oral mucosa, termed smokeless tobacco habits. A dedicated smokeless tobacco user can consume several kilogrammes of tobacco each year in this way. Most smokeless tobacco habits have a limited geographic spread, but international travel and immigration have brought them to new populations and use is increasing dramatically in the USA, with 6% of males being users. Betel quid is widely used in the UK by immigrant communities and further details are given in the section on oral submucous fibrosis. Some smokeless tobacco habits are listed in Table 16.2.
| Name | Habit | Where used | Carcinogenicity in oral mucosa |
|---|---|---|---|
| Chewing tobacco (‘spit’ tobacco) | Chewing a damp plug of cured leaf tobacco or loose strips of leaf. Often flavoured or sweetened | Historically widely used in Europe and USA, now mainly in the USA | Moderate to low for oral cancer |
| Snuff dipping with dry snuff | Placing a pinch of dry snuff in the buccal sulcus | South-eastern USA and Scandinavia | Relatively low. Associated with verrucous rather than squamous carcinomas |
| Betel quid (pan masala; paan) with tobacco | Areca nut, slaked lime, betel vine leaf with or without tobacco, sweeteners, flavourings and spices | Indian subcontinent, SE Asia, Phillipines, New Guinea and China | Very high when tobacco is included. Areca nut is associated with submucous fibrosis in addition |
| Nass | Tobacco, ash, cotton oil quid held in sulcus | Central Asia and Pakistan | High |
| Khaini | Tobacco and lime quid placed in sulcus | India and Pakistan | High |
| Dry (nasal) snuff | Dry snuff inhaled through nose | Now used mainly in Africa | Low, associated with nasal and sinus carcinoma |
| Gutka | Commercially prepared powder of areca nut, tobacco, lime and other flavourings and sweeteners | Indian subcontinent, SE Asia | Probably high, also risk of submucous fibrosis |
| Toombak | Rolled ball of tobacco and sodium bicarbonate placed in sulcus or floor of mouth | Sudan | High |
| Snus (moist snuff sachets; Scandinavian snuff) | Teabag-like pouch of moist unfermented snuff, sometimes with flavouring. Also some rolled leaf products | Originally Scandinavia but now prevalent in the USA | Thought to be low or very low. See section on harm reduction |
Clinical features
Application of tobacco to the mucosa induces mostly hyper-keratotic lesions. Those that use betel quid frequently also may develop erosive lesions or erythroplakia. Lesions are limited to the site where the tobacco is held, usually in the buccal sulcus, and lesions do not have sharply defined margins. These changes seem to take prolonged periods to develop and, in the early stages, there is only erythema and mild, whitish thickening of the epithelium. Later there is extensive white thickening and wrinkling of the mucosa. Betel quid or areca nut users may also develop some localised fibrosis so that the lesions gradually become firm on palpation or submucous fibrosis. Before developing carcinoma, the mucosa will usually show varying degrees of dysplasia on biopsy, but even a non-dysplastic lesion signifies a risk of carcinoma.
Malignant change follows at the site of tobacco placement and only after many years of use. Most habits induce squamous cell carcinoma, but snuff dippers develop verrucous carcinomas (Ch. 17) much more frequently but after a longer exposure. If these remain untreated, invasive squamous carcinoma may develop. Smokeless tobacco products also expose the oesophagus and pharynx to carcinogens and carcinoma may also develop at these sites.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


