Oral Aspects of Metabolic Diseases
The volume of literature pertaining to metabolism is rapidly surpassing the ability of investigators to keep pace with it. Obviously it is impossible to provide an in-depth look at any particular area. Excellent references, many recent and some classic, are available. These include Wolbach and Bessey (1942), Schour and Massler (1943 and 1945), Follis (1948 and 1958), Bodansky and Bodansky (1952), Bourne and Kidder (1953), Duncan (1959), Comar and Bronner (1960), Gÿorgy and Pearson (1967), Sebrell and Harris (1967), Greenberg (1967, 1968, 1969, 1970), Vogel (1971), Hokin (1972), Prasad (1976), Underwood (1977), Stanbury, Wyngaarden, and Fredrickson (1978), Alfin-Slater and Kritchevsky (1979), Goodhart and Shills (1980) and Bondy and Rosenberg (1980).
Disturbances in Mineral Metabolism
Minerals
Calcium
Absorption
Citrates, that may lower the pH of the intestinal tract, form calcium citrate which is relatively soluble. The addition of citrates to a rachitogenic diet seems to render the diet nonrachitogenic and also aid calcification. It has therefore been suggested that the lowering of the intestinal pH aids absorption of calcium and that the calcium citrate ion, though relatively soluble, aids the deposition of calcium in bones by raising the pH of the calcifying tissue or of the fluids surrounding the calcifying tissue. High protein diets have also been shown to increase calcium absorption, probably through the formation of soluble calcium compounds with the amino acids produced by the digestion of the protein.
Phosphorus
Total body phosphorus is approximately 500–800 gm, of which 85–90% is in the skeleton, leaving approximately 100 gm in soft tissues. There are multiple pools of phosphorus having different turnover rates; bones and teeth have the lowest rates. A major portion of phosphorus is incorporated into organic phosphorus compounds (phospholipids of cell membranes, nucleic acids, etc). The normal inorganic phosphate level of blood in adults ranges from 2–4 mg/dl, while in children its range is from 3–5 mg/dl. These blood levels are maintained by a balance of various factors, such as parathyroid hormone, phosphatase activity, and vitamin D. Phosphorus is; however, not as finely regulated as plasma calcium but is under some hormonal control via PTH and renal production of 1,25 (OH)2D3.
Function
When young rats are placed on a low phosphorus diet, there is some retardation in growth. The only specific gross or microscopic alterations are found in the skeletal system, where severe rickets is present (Fig. 15-1). This finding appears after the rats have been on the experimental phosphorus-deficient diet for only one week.
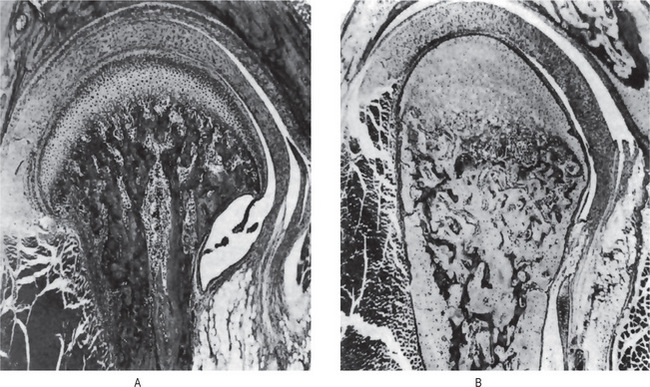
Figure 15-1 Phosphorus deficient diet.
(A) Sagittal section of mandibular joint of a normal rat 70 days of age. (B) Sagittal section of mandibular joint of rat 70 days old, which received a phosphorus-deficient diet since weaning at 21 days of age. Courtesy of Dr Herman Becks
Phosphate depletion in man is nonexistent under most dietary regimens. Long-term antacid use; however, will render phosphate unabsorbable. Lotz and coworkers have described such a condition, which is characterized by weakness, malaise, anorexia, and bone pain. Increased calciuria results in a negative calcium balance with bone demineralization. Rickets and osteomalacia are important dietary deficiency disorders of calcium, phosphorus, or vitamin D. The other causes of hypophosphatemia may be due to decreased intake (starvation, malabsorption, or vomiting) or increased cell uptake as in high dietary carbohydrate, liver disease, or increased excretion due to diuretics, hypomagnesemia, and increased parathormone. Hyperphosphatemia, on the other hand, is due to factitious hemolysis, increased intake of vitamin D, increased release from bone as in malignancy or decreased excretion.
Magnesium
Pathologic Calcification
It is not always possible to make a clear distinction between these various forms.
Dystrophic Calcification
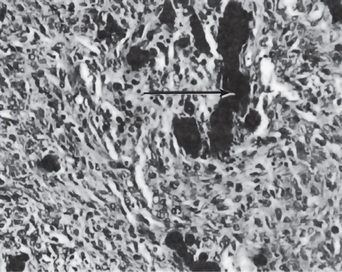
In the mouth, areas of dystrophic calcification may frequently be found in the gingiva, tongue or cheek. Such areas are also found in the benign fibromas of the mouth and adjacent structures (Fig. 15-2). One of the most common intraoral dystrophic calcifications is found in the pulp of teeth, and this has been discussed in Chapter 13 on Regressive Alterations of the Teeth. Boyle described the pulp calcifications as calcific degeneration of the pulp tissue. They are usually found in the teeth of older persons, although they also may be seen in young people. They may occur in the wall of blood vessels or in the perineural connective tissue of the pulp, or they may be rather diffusely scattered both in the pulp chamber and in the root canal. They appear as fine fibrillar calcifications which may coalesce to form large masses of calcific material.
Many of the deposits of calcareous material are found in degenerative processes of the pulp as well as in pulps which are the seat of inflammatory processes. In these cases the calcifications probably have the same relation to body health as calcifications within arterial walls in arteriosclerosis. This type of calcification probably does not cause pulpal inflammation, and there is no justification for considering it a source of dental infection. The other types of pulp stones or pulp nodules (denticles) are discussed in Chapter 13 on Regressive Alterations of the Teeth.
Calcinosis
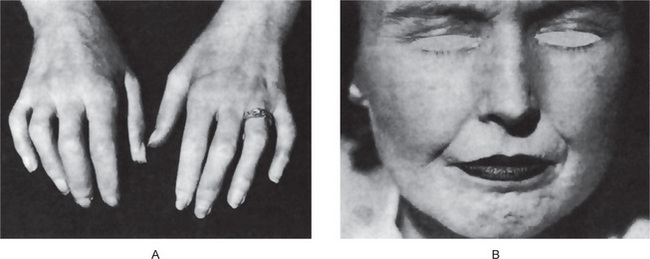
Calcinosis is the presence of calcifications in or under the skin. There are two forms of calcinosis: calcinosis circumscripta, which, as the name suggests, is a circumscribed form, and calcinosis universalis, which is a generalized form. Calcinosis universalis is often associated with scleroderma and sometimes dermatomyositis. These different forms of calcinosis have been discussed by Johnson (Fig. 15-3).
Sodium
The sodium found in the body is mainly associated with chloride and as NaCl and NaHCO3. The sodium ion content of the normal (70 kg) adult male ranges from 83–97 gm. Over one-third of this amount is in the skeleton, of which 65–75% is unexchangeable. Most of the remaining sodium is extracellular and accounts for 90% of the basic ions of both extracellular fluid and plasma. Enamel ash contains about 0.3%. The question of whether the sodium of the dental tissues is associated with the inorganic or organic fractions or with small quantities of tissue fluid present in the teeth remains unanswered.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 . Disturbances in Mineral Metabolism
. Disturbances in Mineral Metabolism . Disturbances in Protein Metabolism
. Disturbances in Protein Metabolism . Individual Amino Acids
. Individual Amino Acids . Lysosomal Storage Diseases
. Lysosomal Storage Diseases . Disturbances in Carbohydrate Metabolism
. Disturbances in Carbohydrate Metabolism . Hurler Syndrome
. Hurler Syndrome . Disturbances in Lipid Metabolism
. Disturbances in Lipid Metabolism . Avitaminoses
. Avitaminoses . Disturbances in Hormone Metabolism
. Disturbances in Hormone Metabolism