15 Low-Level Lasers in Dentistry
Therapeutic Lasers
The simplest way to classify therapeutic lasers is by wavelength. Penetration depths vary; lasers in the red part of the spectrum are more superficially absorbed, whereas IR lasers penetrate as much as 3 to 5 cm, depending on wavelength and target tissue. There is an “optical window” at about 820 nm, which has the greatest depth of optical penetration. Mucosa is quite transparent to the wavelengths (does not absorb light well), skin and bone are fairly transparent, and muscles have the greatest absorption of light. Dosage at the target tissue must be calculated accordingly. Another factor in depth of penetration is the distance from the target tissue, which affects the spot size (see Chapter 2). Irradiation out of contact versus irradiation in contact versus irradiation with pressure on the tissue all deliver different dosages to the tissue. Laser irradiation with tissue pressure causes a slight ischemia in the area, which reduces the hemoglobin concentration in the spot (Figures 15-1 and 15-2).
FIGURE 15-2 • A 650-nm wavelength at only 30 mW shows penetration of low-level laser light through bone.
Mechanisms
The advantage of therapeutic laser light is that it stimulates natural biological processes and mainly affects cells in a decreased oxidation-reduction (redox) reaction. A cell in a low redox stage is acidic, but after laser irradiation the cell becomes more alkaline and able to perform optimally. Healthy cells cannot significantly increase their redox situation and thus will not react strongly to the laser energy, whereas cells in a low redox situation will be stimulated.1,2 The most essential effect may be the increase of adenosine triphosphate (ATP), the “fuel” of the cells, produced in the mitochondria.3 ATP is the end product of the Krebs cycle, where the photon-acceptor enzyme cytochrome-c oxidase is inhibited by nitric oxide (NO). Laser light will dissociate the binding between NO and cytochrome-c oxidase, allowing it to resume ATP production.4 This basic mechanism initiates a cascade of cell signaling, leading to an optimization of body functions.5
Stimulation/Inhibition
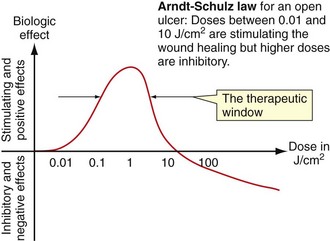
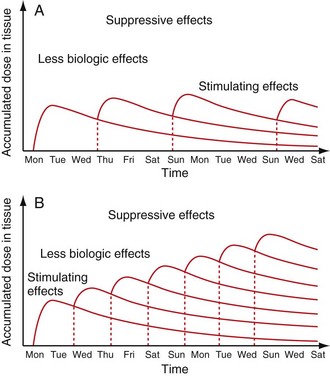
Low-level laser therapy follows the Arndt-Schulz law: too small a stimulus does not trigger any effect. Increased stimulation augments the effect up to an optimal dose level. Increasing the dose even further means that the stimulation is gradually reduced, and at very high doses, stimulation is inhibited. The quest for the “optimal dose” is still not solved, but much is known about “therapeutic windows.” In some patients the goal is inhibition rather than stimulation, especially for pain management. High doses of laser light will inhibit the pain signals, partially by creating transient varicosities along the neurons, impeding signal transmission.6 Opioid-related mechanisms have also been reported,7 as well as a reduction in the compound action potential8 (Figures 15-3 to 15-5).
Acute vs. Chronic conditions
Patients with long-standing chronic pain conditions may experience a flare of pain after LLLT. This is transient and actually shows that the patient is responding well to the treatment. The chronic situation is believed to be transformed into an acute phase, allowing healing to begin. Pain levels are reduced below baseline within 24 hours. The patient should be informed about this possibility before treatment. Other chronic pain patients may respond with deep fatigue, interpreted as an accumulated lack of rest, surfacing when the pain subsides. As with pharmaceuticals, the reaction and dosage must be individualized for each patient (Figure 15-6).
Pulsing
The importance of pulsing the light is obvious when applied to cell monolayers in the laboratory.9–11 The pulse repetition rate (PRR) also helps in the clinical setting, although little is known about pulsing in vivo and animal/clinical studies are inconclusive. It is not yet known how to control these mechanisms through different PRRs. Also, the biological effects of a “chopped” CW laser beam and a superpulsed beam are different. At this time, use of a continuous beam is therefore recommended in units that have CW emission. The 904-nm GaAs laser does not have a CW mode, so a PRR must be selected, relying on anecdotal evidence to select pulse parameters.
Moriyama et al.12 found more expression of the inducible nitric oxide synthase (iNOS) gene after 905-nm superpulsed laser irradiation. This suggests a different mechanism in activating the inflammatory pathway response in superpulsed mode compared with CW mode.
Number of Sessions
Several conditions can be resolved with a single LLLT session, but most conditions need repeated irradiation for optimal results. In the dental situation this is often problematic; patients may be unable to schedule brief laser appointments. Typically, the laser is used whenever the patient comes for routine dental treatment, such as for prophylaxis or cavity preparation. As a result, laser therapy might not be optimized. In many cases the irradiation can be delegated to the assistant or hygienist. In some situations the patient can borrow, rent, or buy a simple low-power laser (even a traditional 5-mW laser pointer) for a period to optimize the treatment by applying daily doses according to the dentist’s instructions (Figure 15-7).
Side Effects and Contraindications
Because LLLT affects blood flow in undefined ways, irradiation of patients with coagulation disorders should be avoided.13,14 Presence of known malignancies is another contraindication, because LLLT stimulates cell growth. The literature also discusses pregnancy as a contraindication,15 although dentists work exclusively in the oral and head/neck regions. Also, although sometimes listed as a contraindication, pacemakers are electrical and not influenced by light. Some traditional safety regulations and contraindications seem to have been transferred from electrosurgical and other therapies to surgical lasers.
A contraindication especially relevant to dentistry is irradiation over the thyroid gland, located within the dental treatment area. Dentists generally are not informed about possible hyperthyroid or hypothyroid conditions, so direct irradiation over this area should be avoided. However, LLLT for thyroid disorders has been studied.16
Documentation
Because of the complex mechanisms behind LLLT, the literature is still not at an evidence-based level, except for a few indications. Still, more than 100 dental institutions worldwide are represented in the literature, comprising more than 3000 studies. Annually, about 250 papers on LLLT appear on PubMed, many on dental issues and most reporting positive results. Reports of no effect from LLLT can often be attributed to very low doses, miscalculation of the supposed dose, and ineffective treatment applications.17 Nevertheless, some qualified negative studies underline that LLLT is not a “hit and run” therapy but depends on knowledge of all the parameters. A correct diagnosis and sufficient dosage are key to success.
Laser Safety
It is prudent to use protective goggles that are wavelength specific. Most therapeutic lasers have divergent beams, so from a distance of only a few centimeters, the intensity (and danger) is considerably reduced. LLLT has even been successfully used to treat macular degeneration,18 affirming the importance of wavelength and intensity. Some LLLT lasers have a collimated lens system, producing a parallel beam. These have no advantages in dental LLLT, and when used in contact with tissue, the effect of the collimation is lost.
Choosing the “Right” Laser
Power and Time
During the past decade, stronger lasers have been advocated as more effective, and thus lasers with output of 500 mW or more are marketed. For musculoskeletal conditions and pain therapy, high-output lasers may be useful, but for processes such as wound healing and bone regeneration, lower power over a longer time is more effective. Availability of power adjustments in a therapeutic laser, as with a surgical laser, is therefore helpful. Applying 5 J in 10 seconds with a 500-mW laser is quite different than applying the same 5 J with a 50-mW laser in 100 seconds. The 500-mW laser would work better for pain but might be less effective for treatment involving tissue regeneration.19 This reemphasizes why training is so important in the decision-making process.
Biostimulation
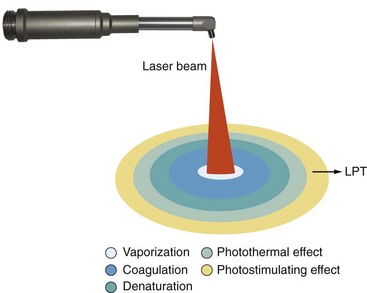
Although dental lasers such as the neodymium-doped yttrium-aluminum-garnet (Nd:YAG), carbon dioxide (CO2), and erbium family are considered “hard” or “surgical” lasers, they may be producing a degree of biostimulation in the areas peripheral to the focal spot, where the energy is reduced to stimulatory levels (Figure 15-8).
Some of the positive effects observed with the hard lasers may be explained by biostimulation. Pourzarandian et al.20,21 reported stimulation of human gingival fibroblasts by low-level Er:YAG irradiation, as well as increased prostaglandin E2 (PGE2) production through induction of cyclooxygenase-2 (COX-2) messenger ribonucleic acid (mRNA) in human gingival fibroblasts. Additionally, Er:YAG lasers may be used at lowest output and scanned at a slight distance (out of focus) to produce biostimulation. The problem with using these hard lasers in this manner is that no microprocessor informs the practitioner how to control the dosage, and the fibers are not adapted for biostimulation.
Biostimulation is not limited to the traditional near-IR wavelength window; such effects are even reported when using defocused CO2 lasers.22–24 The CO2 laser has an extremely poor penetration through tissue because it is so well absorbed at the surface, and the biological effects reported on deeper tissues may first appear improbable. However, the coherent light is absorbed in peripheral microvessels, and the clinical effect observed shows that LLLT has primary effects at the target as well as systemic effects through blood and lymph circulation. Thus, with minimal calculation, the owner of a “hard” laser can have a “soft” laser for free. Least complicated is the surgical diode laser, with simpler calculations and wavelengths within the traditional range of biostimulation.
Photoactivated Disinfection
The photoactivated disinfection (PAD) method is already commercialized and recommended for use in periodontal pockets, periimplantitis, deep carious lesions, and infected root canals.24–28 The laser must work within the absorption range of the dye used and is generally in the red spectrum, with a power output of 50 to 100 mW. The selected dye is applied and allowed to diffuse for a few minutes, and then the laser is used. Some transient discoloration may be seen in dentinal and mucosal areas.
Curing Light
In a 2009 in vitro study, Enwemeka et al.29 demonstrated that light-emitting diode (LED) energy with a 470-nm peak successfully kills methicillin-resistant Staphylococcus aureus (MRSA). Irradiation produced a statistically significant, dose-dependent reduction in both the number and the aggregate area of colonies formed by each strain of cells. The higher the dose, the more bacteria were killed, but the effect was not linear, being more impressive at lower than at higher doses. Almost 30% of both strains were killed with as little as 3 J/cm2. As much as 90.4% of the two different colonies were killed with an energy density of 55 J/cm2. This study requires further in vivo investigation but suggests an innovative use of an available dental light source to treat MRSA infection, which has high morbidity and high mortality.
Noncoherent Light
Blue light, as in curing lights, has a bactericidal effect in general. Importantly, curing lights are noncoherent, whereas lasers are coherent. Whether or not coherence is important has long been discussed, and some claim coherence is not needed. This misunderstanding is based on in vitro studies using cell monolayer that emphasize wavelength and intensity, as discussed earlier.5 The in vivo situation is different, however, and thus far a relative equivalence between coherent and noncoherent light has only been documented for superficial conditions such as open wounds.
Acupuncture
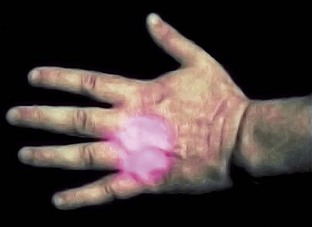
Few dentists use acupuncture and, unless trained, should avoid it. However, the therapeutic laser seems to produce effects similar to those of acupuncture needles, with some safe points to use. For example, P6 (Neiguan) on the wrist is an excellent point to reduce nausea and vomiting.30 The location of this point is 2.5 cm up from the wrist and 0.5 to 1.0 cm deep. The point is between two tendons. Delivery of 3 to 4 J to this point often allows a more relaxed environment for taking impressions or working in the molar area, especially in patients with a heightened gag reflex. Another easily accessible point is the Li4 (Hegu), a pain-reducing point located in the middle of the second metacarpal bone on the radial side (Figure 15-9).
Functional magnetic resonance imaging (fMRI) has confirmed the similarity between needles and lasers on the same acupuncture point.31 However, understanding this phenomenon becomes more difficult because there is no “qi” effect with the laser. The “gate control” theory would not explain the effects because there is no pain stimulus in the acupuncture point when using a laser.
Dental Indications
Anesthesia
Applying LLLT to the mucosa before an injection results in a slight anesthetic effect, although this cannot be obtained in the hard palate.32 LLLT applied before the injection will also improve healing, should the needle cause trauma to a vessel or nerve. LLLT improves local microcirculation,33,34 so the effect of the numbness can be shortened if LLLT is applied to the site after completing the dental procedure. From 4 to 6 J is needed in both cases.
Aphthous Ulcers
The healing time of aphthous ulcers can be shortened and the immediate pain reduced by administering 4 to 6 J over the lesion.35,36 LLLT does not seem to be as effective for aphthous ulcers as the more aggressive approach using surgical lasers (see Chapter 6). Patients with aphthous ulcers should avoid toothpastes containing sodium lauryl sulfate, which may trigger these lesions in predisposed persons.
Edema
The lymphatic system plays an important role in the inflammatory process, and LLLT over the involved lymph nodes decreases edema. Start irradiation over the most distal nodes of the chain involved, and work toward the focus of the swelling, using 2 to 3 J per node. Reduction of large edemas requires high doses locally, preferably by IR low-level laser units. The permeability of the lymph vessels will be reduced, lumen size increased, and repeated irradiation will stimulate growth of collaterals37–41 (Figure 15-10). The lymphatic system brings the lymphocytes and natural killer cells to fight the infection. Meneguzzo et al.41 reported that an 810-nm laser reduced rat paw edema whether the irradiation was performed in the paw itself or over the inguinal lymph nodes.
Endodontics
Sousa et al.42 analyzed the effect of LLLT on the secretory activity of macrophages activated by interferon gamma (IFN-γ) and lipopolysaccharide (LPS) and stimulated by substances leached from an epoxy resin–based sealer (AH-Plus) and a calcium hydroxide sealer (Sealapex). The production of tumor necrosis factor alpha (TNF-α) was significantly decreased by LLLT, regardless of experimental group. The level of secretion of matrix metalloproteinase-1 (MMP-1) was similar in all groups.
Laser application after overinstrumentation and overfilling is a good example of an indication for LLLT in endodontics. Because LLLT can stimulate bone formation, apical bone resorption probably can heal faster after completed endodontic treatment if LLLT is applied. Energy needed is related to the depth of the apex, ranging from 4 to 8 J per apex. For the same reason, intraoperative and postoperative LLLT in apical surgery has potential,43,44 but solid scientific documentation is still lacking. However, irradiation over the suture line will stimulate fibroblast proliferation and increase tensile strength.45
In patients with acute pulpitis, when the affected tooth or root is difficult to pinpoint, the laser can be applied over the apices in the involved area. The affected tooth may react by a pain increase, probably because of increased microcirculatory pressure in the pulpal chamber. LLLT can also be used as an adjunct therapy in pulp capping46,47 and pulpotomy48,49 (see Chapter 12). In both cases the exposed pulp is irradiated at low intensities, applying 2 to 3 J before traditional methods are used. The irradiation will reduce inflammation, preserve odontoblastic integrity, and stimulate cellular proliferation.
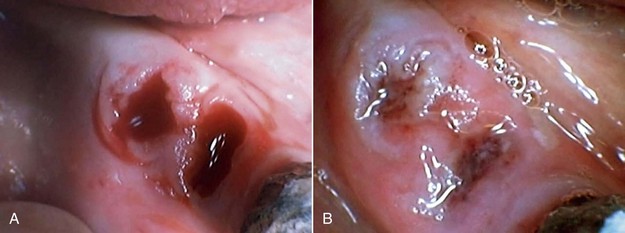
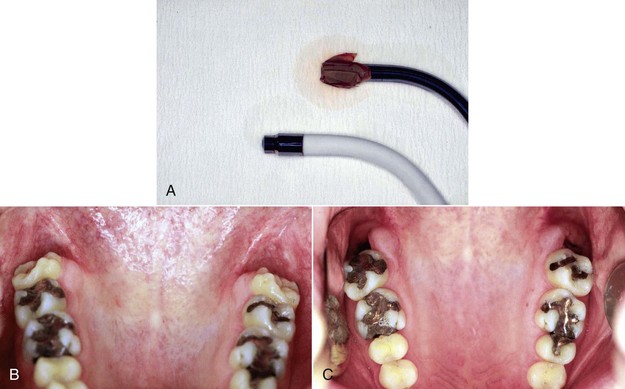
Extractions
Nontraumatic approaches and good postoperative procedures are the key to satisfactory healing after extractions. However, the occasional complication is unavoidable. Adding LLLT after the extraction will reduce the inflammatory phase, induce pain reduction, stimulate the fibroblasts in the wound periphery, and stimulate the osteoblasts in the socket.50–55 High doses of laser energy can focus on the postoperative pain, but to obtain a good healing process, lower power and longer time are more advantageous.
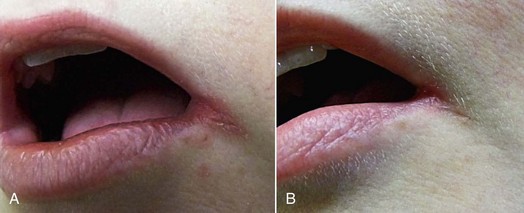
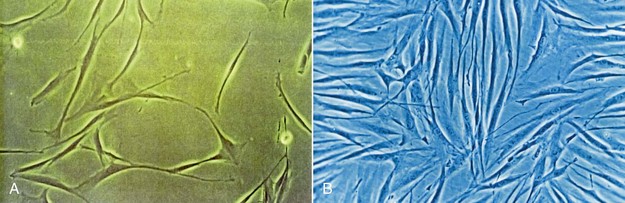
The major goal of LLLT after extractions is to stimulate the fibroblasts to seal the socket. In cases of failure (dry socket), the traditional methods are used in combination with high doses of LLLT to reduce patient discomfort. When the dressing is changed during subsequent appointments, lower doses are given to stimulate fibroblast growth. Not only does LLLT stimulate fibroblast proliferation, but the cells are arranged in parallel bundles as well, creating a smoother area.2 This is of particular interest in extraoral surgery when the cosmetic aspect is important (Figures 15-11 to 15-13).
Herpes Simplex Virus
Patients with a herpes simplex virus type 1 (HSV-1) eruption may be reluctant to visit the dentist. However, LLLT is the most efficient method of treating this infection.36,56–58 In particular, if treated during the initial prodromal stage (when the patient feels the initial tingling), the healing will only take a few days or may even disappear within hours. Importantly, patients with recurrent HSV-1 attacks will experience longer intervals between the outbursts. Large blisters can be opened with surgical lasers (e.g., erbium, CO2) to empty the fluid/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses