CHAPTER 15 Dental Biomaterials
CHARACTERISTICS OF DENTAL MATERIALS
Understanding of mechanical, physical, electrical, surface, biological properties of dental materials enables clinician to determine proper use and care of dental materials.
Mechanical Properties
A. Forces:
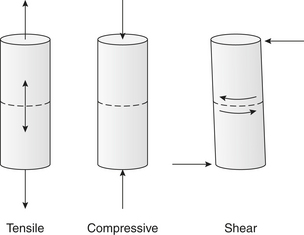
B. Stress: reaction with which object resists external force (Figure 15-1). Example: stretching of orthodontic band causes stress because wire is being pulled in tension; can resist this external force up to point of fracture.
C. Strain: deformation; change in length or dimension of a dental material because of applied stress.
1. Deformation or change in length (inches per meter)/Original length (inches per meter). Example: dental waxes exhibit strain much MORE quickly than amalgam because of differences in stiffness.
3. Strain-related properties:
b. Plastic deformation: permanent strain or change in shape of object that results when stressed beyond elastic limit.
d. Ductility: ability of a material to withstand significant plastic or permanent deformation under stress before fracturing (e.g., gold), opposite of brittleness.
D. Elasticity: permits material to be deformed by applied load and then assume original shape when load is removed (just like Gumby and Pokey). Examples: orthodontic wires resist fracture upon manipulation by clinician; adjustment of clasps on partial dentures; ability of fixed partial denture (dental bridge) to withstand forces of mastication.
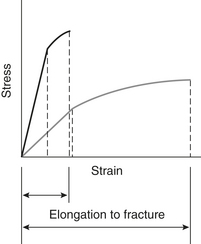
1. Modulus of elasticity: measure of stiffness of material and ability to resist bending or change in shape.
2. Stress/strain curve: graphically shows change in dimension for given application and identifies important points such as elastic limit, proportional limit, and permanent deformation; used in dental research (Figure 15-2).
E. Hardness: resistance of a solid to penetration. Example: clinician must instruct denture patients NOT to use abrasive cleansing materials, such as a paste with grit having higher hardness than denture, to prevent excessive abrasion of denture because of its low abrasion resistance.
| Dental material | KHN |
|---|---|
| Acrylic | 20 |
| Dentin | 70 |
| Calculus on teeth | 86 |
| Enamel | 350 |
| Porcelain | 400-500 |
KHN, Knoop hardness number.
CLINICAL STUDY
| Age | 59 YRS | SCENARIO |
| Sex |  Male Male  Female Female |
The patient is in the dental office because of a fractured tooth. Visual examination of tooth #18 reveals a fracture of the ML cusp. The remainder of the tooth holds a large MOD (mesio-occlusal-distal) amalgam restoration. After the dentist removes all of the old amalgam, some caries is discovered that will result in a near pulpal exposure. Patient also has amalgam restorations in teeth #2, #14, #19, and #31, and full gold crowns on teeth #3 and #30. |
| Chief Complaint | “I bit down on a popcorn kernel and my tooth broke.” | |
| Medical History |
2. The dentist decides to place a cavity liner before restoring this tooth. Explain what material would be appropriate and why.
3. How should this tooth be restored? Select an appropriate restorative material, and include indications and contraindications for different restorative materials.
1. Stress from biting on the popcorn kernel created both compressive and tensile forces on the tooth; smaller the area over which a force is applied, the larger the value of stress. In this instance, occlusal surface of #18, from contact with the kernel, magnified the stress. Second property involved was strain (reaction of tooth #18 to biting stress), which resulted in fracture of ML cusp.
2. Calcium hydroxide is appropriate material for lining the cavity. Advantages of material: (1) encourages recovery of the pulp by stimulation of secondary and reparative dentin; (2) protects pulp; (3) sufficiently strong. Mineral trioxide aggregate (MTA) may also be effective for lining the cavity.
3. Tooth #18 will probably require full-coverage restoration because of extensive loss of tooth structure. Because the tooth that opposes #18 has never been restored, full gold crown is indicated; gold would cause minimal abrasion of #15. Porcelain would be contraindicated in this circumstance because has high hardness number (KHN 400-500) compared with enamel (KHN 350) and would therefore result in the eventual abrasion of tooth #15.
Physical Properties
A. Thermal properties: reaction to temperature changes within oral cavity and subsequent expansion and contraction. Example: rapid change in oral temperature would occur when eating ice cream cone immediately before drinking cup of hot coffee and affect restorations by expansion.
1. Coefficient of thermal expansion: value or measurement of change in length per unit length for each degree of temperature change (change in volume in relationship to change in temperature).
C. Electrical properties: generation of electrical currents through a variety of means.
D. Color properties: give restorations and prosthetic appliances appearance of natural teeth and soft tissues (esthetics); important in selecting tooth-colored restorations and calibrating whitening procedures (digital machines available).
E. Translucency: how light enters the tooth; affected in several ways:
1. Part of the light may be transmitted completely through the tooth (the MORE light that is transmitted through, the MORE translucent the material).
2. Part of the light may be reflected from the surface of the tooth and thus may NOT penetrate at all.
3. Part of the light may enter the tooth and then scatter and be absorbed (refraction).
Table 15-2 Expansion of tooth and dental materials: linear thermal coefficients of expansion
| Material | Coefficient (×10-6/°C) |
|---|---|
| Tooth | 11.4 |
| Amalgam | 25.0 |
| Acrylic resin | 81.0 |
| Composite resin | 35.0 |
| Silicone impression material | 210.0 |
Surface Properties
A. Solids: MOST materials in dentistry.
B. Liquids: some dental materials are liquids at some point and then change to solids by cooling or chemical reactions.
C. Attaching solid structures to each other: by mechanical bonding, cohesion, adhesion.
1. Mechanical bonding:
2. Cohesion: force of attraction between like kinds of atoms and molecules within material, resulting in tenacious bond. Example: cohesive forces that hold cement together and prevent fracture within cement.
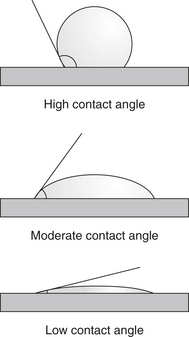
3. Adhesion: force of attraction between unlike atoms and molecules on two different surfaces when brought into contact.
c. Effectiveness of adhesion is determined as follows (Figure 15-4):
d. Inhibition or failure of adhesion is affected by:
(1) Dirty or contaminated surfaces: result in reduced surface energy of adherent, thus reducing ability to attract other atoms and molecules.
Biological Properties
A. Allergic response (immediate or delayed contact allergic reaction type):
B. Microleakage:
IMPRESSION MATERIALS
A. Classification of impression materials:
1. Inelastic materials: rigid impression materials are restricted to applications in which NO undercuts exist.
B. Composition and chemistry:
2. ZOE impression paste: supplied as two-paste system: zinc oxide, eugenol, oils (such as oil of cloves), resin, additives.
C. Characteristics, properties, manipulation of each:
3. Irreversible hydrocolloid (alginate):
a. Suspension of molecules (colloid) in some type of dispersion medium (water); a liquid colloid is a sol.
b. Has 1.5% permanent deformation.
(2) Results in increased tear and compressive strength if impression is left in mouth until fully set.
c. Procedure for use:
(1) Mixing:
(a) Fluff alginate powder container or package; measure appropriate amount of cool water for required number of scoops of alginate; place water in plastic bowl.
(2) Filling and seating the tray:
4. Reversible hydrocolloid (agar): sol is changed into gel by physical (cooling) reaction and therefore is reversible; gives highly accurate impression; MUST be poured immediately to avoid dimensional changes.
5. Polysulfide: provides MOST reproduction of surface detail.
6. Silicone rubber:
b. Addition (polyvinylsiloxane) type:
(1) Low dimension change of 0.1%; does NOT require immediate pouring, can be delayed for up to 7 days.
(2) Lowest level of permanent deformation of ALL elastomeric impression materials on removal from mouth.
D. Clinical uses:
1. Compound:
2. ZOE impression paste: cementing media; also is used for surgical dressings (after periodontal surgery), temporary restorations, root canal filling material, bite registration paste, impression material for dentures.
3. Irreversible hydrocolloid:
4. Reversible hydrocolloid: SAME uses as irreversible hydrocolloid but produces MORE accurate detail.
Table 15-3 Composition of irreversible hydrocolloid alginate impression material
| Ingredient | Percentage (%) | Function |
|---|---|---|
| Diatomaceous earth | 60 | Filler |
| Calcium sulfate | 16 | Reacts with potassium alginate to create gelation |
| Potassium alginate | 15 | Reacts with calcium sulfate to create gelation |
| Zinc oxide | 4 | Filler |
| Potassium titanium fluoride | 3 | Accelerates setting of gypsum |
| Trisodium phosphate | 2 | Retardant |
Table 15-4 Composition of reversible hydrocolloid
| Ingredient | Percentage (%) | Function |
|---|---|---|
| Agar-agar | 8-15 | Substance is extracted from a type of seaweed whose fibrils form a colloid that results in a partially rigid but elastic gel |
| Water | 80-85 | Main component that occupies the spaces between the agar-agar fibrils |
| Borax | Trace amounts | Increases the strength of the gel (also can retard the setting of gypsum; requires the addition of an accelerator) |
| Potassium sulfate | 2 | Accelerates the setting of gypsum |
Replication Materials
B. Types:
1. Model plaster (type II): heating gypsum under atmospheric pressure results in a porous, irregularly shaped material.
C. Characteristics and properties:
1. Hemihydrate: physical differences in hemihydrate particles determine manipulative properties for mixing particles and use of each.
D. Types and uses of gypsum:
2. Model plaster (type II): weakest; lowest compressive strength because of excess water, higher W/P ratio; NOT used intraorally; makes study casts (models) for documentation:
a. To permit clinicians to examine conditions in the mouth from all views; allows study of relationships between adjacent and opposing teeth and permits dentist to examine, measure, and analyze without discomfort to the patient.
b. Beginning of treatment and progress of involved or long-term treatment; for drawing, completing diagnostic waxing, or performing proposed treatment on models.
4. High-strength dental (die) stone (type IV [low expansion] or V [high expansion]):
E. Procedure for use:
1. Dispensing: weigh powder and vacuum mix; water should be measured in graduated cylinder to get consistent results; dimensional accuracy increased when gypsum is weighed and water is measured.
2. Mixing: performed in a rubber bowl, using a stiff metal spatula, with room-temperature water.
b. Spatulate vigorously for 30 to 60 seconds to break up clumps and enhance wetting of powder, strop (wipe) against side of the bowl.
3. Pouring:
b. Plaster or stone is dripped into one corner of the impression and gently vibrated to slowly move the mix around the arch and express any air to coat impression.
c. Second increment is added and vibrated; excessive vibration SHOULD be avoided because it creates air bubbles in the material.
| Gypsum | Water (mL/100 g powder) |
|---|---|
| Model plaster (type II) | 37-50 |
| Dental stone (type III) | 28-32 |
| High-strength dental stone (type IV-V) | 19-24 |
∗ Recommended W/P ratios vary among products and manufacturers.
DIRECT RESTORATIVE MATERIALS
Esthetic Restorations
Esthetic (tooth-colored) restorations include ceramics, porcelains, composites.
A. Ceramics: compounds formed by union of metallic oxides and minerals.
2. Glass ceramics are used MAINLY as reinforcing agents and fillers for dental composites and routinely as coatings and veneers to improve esthetics of metallic dental restorations; also in several dental cements and temporary restorations.
B. Porcelains: specific type of ceramic that is used extensively in dentistry; fabricated by dental technician.
1. Chosen because of esthetic qualities; matches adjacent tooth structure in color, intensity, translucence.
4. High hardness number (Mohs 6 to 7, KHN 400-500); may cause rapid abrasion of opposing enamel, which has lower hardness number (Mohs 5 to 6; KHN 350).
C. Composites: used in ALL classes of restorations, as veneers to cover teeth stained by drugs and chemicals such as fluoride or tetracyclines, to fill spaces between teeth (diastemas), to enhance contour of misshapen teeth.
3. Polymerization: chemical reaction that converts small, individual monomer molecules into long, giant polymer molecules.
a. Consists of activated two containers of composite paste: one contains a benzoyl peroxide initiator, other contains tertiary amine activator.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 •
•