Chapter 14 Bleaching
Section A Bleaching and Its Relevance to Esthetic Dentistry
Brief History of Clinical Development and Evolution of the Procedure
Some of the more arcane bleaching proposals at the turn of the century included electric currents and ultraviolet waves (Rosenthal). Obviously, neither of these really caught on with the mainstream dentist. Acid dissolution of brown fluoride stains was yet another approach to discoloration. This technique was first documented by Kane in 1916. The technique involved the use of 18% HCI to dissolve the superficial layers of enamel. Further investigations were conducted by McCloskey in 1984. In 1918, Abbot pioneered the whitening effects of Superoxol (Figure 14-1). He found that although the chemical was suitable for bleaching teeth, its activity could be enhanced by the addition of heat and light. Some current bleaching techniques are based on Kane and Abbot’s developments.

FIGURE 14-1 Superoxol, a whitening agent whose effects were pioneered by Abbot in 1918.
(Permission granted by Integra Miltex, a business of Integra LifeSciences Corporation, Plainsboro, New Jersey.)
It is only in the last two decades that dentistry has finally begun to provide patients with reasonable methods for vital tooth color de-staining. In 1989 a new procedure was developed (Figure 14-2) whereby a stabilized solution of carbamide peroxide or perhydrol-urea was placed into a custom molded tray, which the patient was required to place over the teeth for hours at a time. This gentle solution worked to gradually whiten the teeth in a much more predictable, safer manner than the earlier bleaching methods.
Safe vital whitening requires an activating material that is acceptable to both the hard and soft tissues, one that is both non-caustic and non-toxic. Feinman, in discussing peroxide-heat-light bleaching procedures, stated that bleaching vital teeth was more difficult than treating non-vital ones. With the more recent, less caustic tooth whitening techniques, precisely the opposite is now true. It is not only much easier to whiten vital teeth than non-vital ones, it is even easier to whiten the entire arch than to work with a single discolored tooth. This paradigm shift alone may account for the immediate acceptance of at-home tooth whitening by the dental community (Box 14-1).
Box 14.1 Tooth Whitening Timeline
Initial Attempts at Bleaching
| 1877 | Chapple—oxalic acid |
| 1888 | Taft—calcium hypochlorite |
| 1884 | Harlan—hydrogen dioxide |
| 1895 | Electrical currents |
Non-Vital Bleaching Initiated
| 1895 | Garretson |
| 1911 | Rosenthal—ultraviolet waves |
| 1916 | Kane—18% hydrochloric acid |
Successful Non-Vital Bleaching
| 1958 | Pearson—intrapulpal bleach |
| 1967 | Nutting and Poe—walking bleach |
| 1978 | Superoxol heat and light |
Modern Techniques
| 1989 | Munro—outpatient tooth whitening |
| 1990s | General use—in-office vital bleaching |
| 1995 | Yarborough—laser-assisted beaching |
The percentage of hydrogen peroxide in gel or liquid form was also increased. The problem with this innovation was that hydrogen peroxide, even in low concentrations such as 10%, can be quite caustic to the soft tissues. Although it does not appreciably affect the hard tissues, it can create peroxide burns on the gingiva or papillae and nearby oral soft tissues. Thus, application of higher percentage peroxides, up to 35% or 50%, required an effective paint-on rubber dam barrier to protect the gingiva and the oral tissues. Regular rubber dams allowed liquid peroxides to seep between the teeth and the dam and burn the peridental soft tissues. Protective gels were often applied to the soft tissues as well, but some of the higher-concentration peroxides still managed to cause damage. The paint-on rubber dams (Figure 14-3) (Pulpdent Kool-Dam paint-on dam) offered protection for the lips, cheeks, and face.
Clinical Considerations
Contraindications
Differentiating Stains
Differentiating the quality and cause of stains is of more than merely academic interest. Knowing what caused the dental staining allows the dentist to better plan the whitening technique and to provide a more accurate prediction of the outcome. Staining and discoloration of the teeth can be caused by many factors. Traditionally, tooth discolorations are divided into extrinsic and intrinsic categories (Box 14-2).
Intrinsic Stains
Intrinsic Discolorations Created during Odontogenesis
Amelogenesis imperfecta (Figure 14-4) is considered a genetic defect that can affect both the primary and the permanent dentitions. The most common modes of inheritance are either autosomal recessive or autosomal dominant. Three categories have been identified: hypomaturation, hypocalcific, and hypoplastic. These display considerable differences in appearance both within and among groups. Hypomaturation has an autosomal dominant mode of inheritance, and presents as enamel that has chipped away from the underlying dentin. Hypocalcific cases exhibit enamel that has normal thickness but is soft. The enamel is often completely abraded away soon after eruption. The tooth crown ranges in appearance from a dull opaque white to a dark brown. These teeth are also have a rough and pitted surface. Hypoplastic teeth have enamel that is quite thin, often to the point where interproximal contacts are eliminateed. Hypoplastic teeth have a smooth, hard, yellow appearance, with pitting found on occasion.
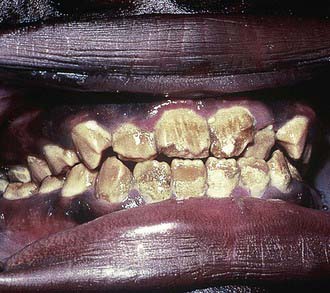
FIGURE 14-4 Amelogenesis imperfecta, hypocalcified type.
(From Pinkham J, Casamassimo P, Fields H, et al: Pediatric dentistry: infancy through adolescence, ed 4, St Louis, 2006, Mosby.)
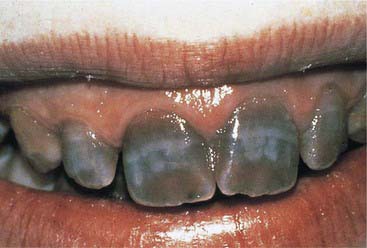
Dentinogenesis imperfecta (Figure 14-5) is an inherited trait that is the most prevalent hereditary dystrophy affecting tooth structure. Typically seen more severely in the primary dentition, the clinical crowns appear reddish-brown to gray opalescent. The enamel is often friable and breaks off soon after eruption. The exposed softened dentin rapidly abrades away. The thin or nonexistent enamel makes full prosthetic coverage the only viable treatment option. Vital bleaching is contraindicated.
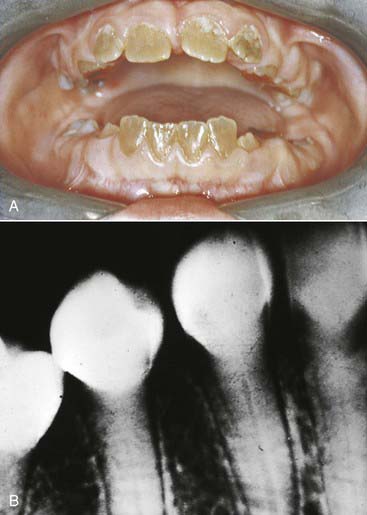
FIGURE 14-5 (A) Clinical and (B) radiographic appearance of dentinogenesis imperfecta.
(From Ibsen O, Phelan J: Oral pathology for the dental hygienist, ed 5, St Louis, 2009, Saunders.)
Endemic fluorosis (Figure 14-6) is an enamel discoloration caused by excessive intake of fluoride during odontogenesis. Fluorosed teeth range from slight wisps or flecks of opaque white to mottled or pitted darkened surfaces. The condition was described as early as 1916, although the causative agent was not identified until 1931. Black thought the stain was caused by replacement of the normal cementing substance between enamel rods by a material that he named “brownin.” It is now known that dental fluorosis is a form of enamel hypoplasia, resulting from metabolic alteration of the ameloblast during enamel formation. Dental fluorosis is often found in communities where the fluoride content of the drinking water exceeds 1 part per million. The severity of the staining is directly proportional to the amount of fluoride absorbed. The teeth can be affected from the second trimester in utero through age 9 years.
Porphyria (Figure 14-7) is a porphyrin metabolism disorder that results in increased formation and excretion of porphyrins. It is usually genetically transmitted and rare, but may develop later in life. Neurological, psychological, and gastrointestinal symptoms may present as well. The hematoporphyrin pigment causes a characteristic reddish-brown discoloration of the teeth (erythrodontia). The dental effects are more common in the primary than the permanent dentition. Coloration is dispersed throughout the enamel, dentin, and cementum, and fluoresces under ultraviolet light. Tooth whitening, and possibly bonding, can be effective.
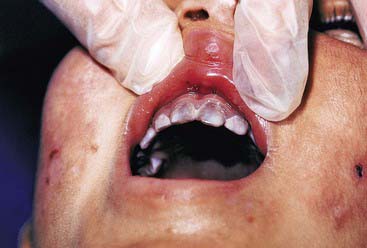
FIGURE 14-7 Congenital erythropoietic porphyria. Brownish teeth fluoresce under Wood lamp examination.
(From Kliegman R, Behrman R, Jenson H, Stanton B: Nelson textbook of pediatrics, ed 18, St Louis, 2008, Saunders.)
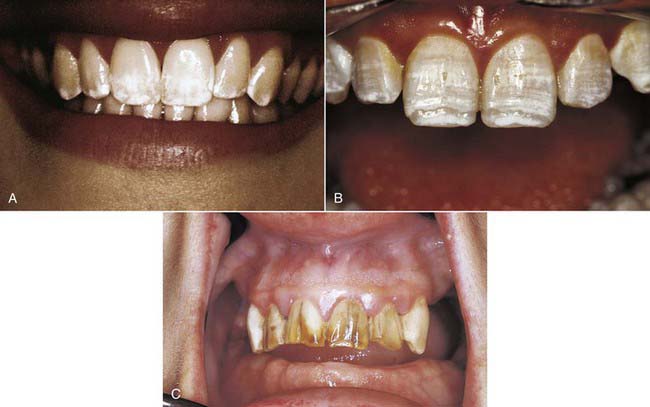
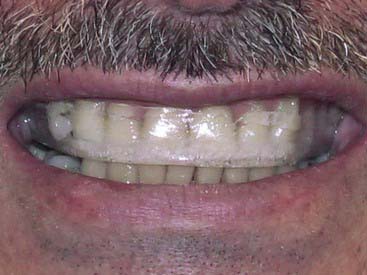
The potential for tetracycline to cause discoloration (Figure 14-8) of the dentition is well documented and studied since it was first reported by Schwachman and Schuster in 1956. Because tetracycline can cross the placental barrier, tetracycline affects both the deciduous and permanent dentitions, making the teeth vulnerable throughout odontogenesis. Even an exposure as short as 3 days can cause discoloration of the teeth at any time between 4 months in utero and age 9 years. The mechanism of the staining caused by tetracyclines is related to the calcium binding in the tooth. Tetracycline binds to the tooth calcium, forming a tetracycline–calcium phosphate complex. It occurs throughout the tooth but is most highly concentrated in the dentin near the dentino-enamel junction. Both the quality and the severity of the discoloration are directly related to the specific tetracycline ingested as well as the dose. Some early investigations revealed that teeth affected by tetracycline first exhibit a yellow coloration and a bright yellow fluorescence that differs significantly from the blue fluorescence of normal, healthy teeth. The color of the affected teeth gradually changes over the succeeding months or years. The shade change is most noticeable in those teeth that are most exposed to extraoral light—specifically, the facial surfaces of the anterior teeth. Wallman and Hilton clearly demonstrated the role of light in this process in 1962 by splitting a tetracycline-stained tooth lengthwise and exposing only one half to light. The light-exposed half underwent a color change to brown, whereas the unexposed half remained yellow. For this reason many researchers believe that the use of heat and light bleaching systems to treat tetracycline stains may be contraindicated.
Post-Eruptive Discoloration
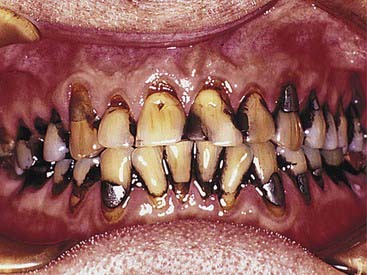
Dental metals are the most ubiquitous source of staining—specifically, leeching of amalgam corrosion products (Figure 14-9). Threaded stainless steel pins or gold-plated retentive pins can cause similar extremely dark stains that pose significant challenges for any whitening effort.
Material Options
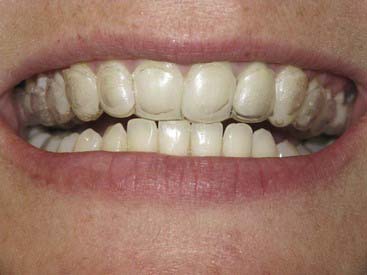
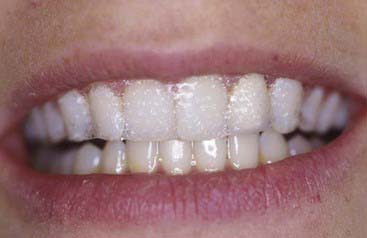
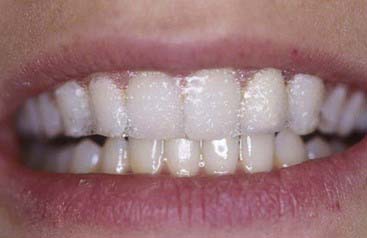
The material options for at-home bleaching include bleaching trays. In most cases a custom-made tray is fabricated by the dental office or laboratory and given to the patient (Figure 14-10). The patient injects the bleaching agent into the tray during the day, overnight, or both, and inserts the tray over the teeth; treatment for an entire arch (or both arches) typically requires about 2 to 4 weeks. At-home tray-less techniques are similar; no prefabricated or custom-fabricated trays are needed. Double-tray systems, such as Opalescence Trèswhite Supreme (Ultradent Products, Inc., South Jordan, Utah), have an inner, softer tray pre-loaded with the bleaching gel and an outer harder tray that is used to position the entire system over the teeth (Figure 14-11). This technique does not require the in-office fabrication of a tray and thus offers time advantages for most practices. Bleaching strips without trays, such as Crest 3D White Whitestrips (Procter & Gamble, Cincinnati, Ohio), are placed over the teeth and manually adapted to the tooth anatomy (Figure 14-12). The patient pats the strips onto the tooth surfaces and leaves them in place for about 30 minutes per application (Figure 14-13).
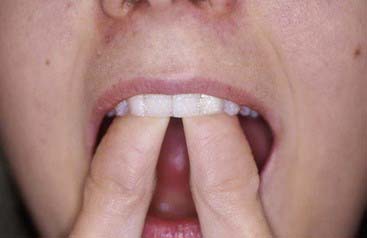
FIGURE 14-12 Bleaching strips are placed over the teeth and adapted to the teeth with the patient’s fingers.
Tray Bleaching Systems
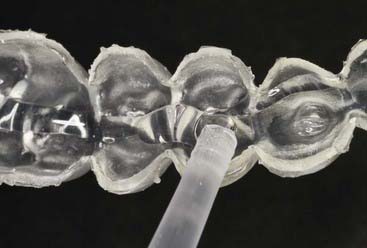
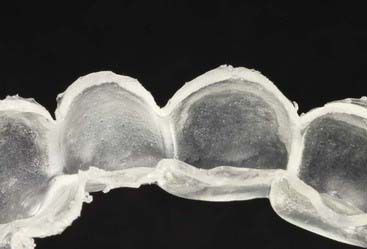
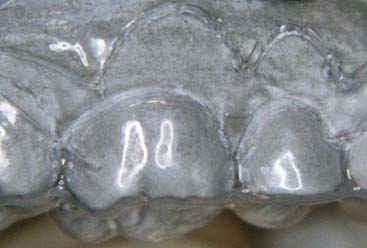
Advantages of the tray system include the predictable volume (Figure 14-14) of the bleach applied to the teeth and the ability to effectively spread the bleach to every tooth, covering their occlusal, buccal, lingual and interproximal aspects as well. The tray can be comfortably worn for several hours to overnight, even though the efficacy of the bleaching gel decreases progressively and the material becomes inactive for bleaching after 3 to 4 hours. Most of the bleaching effect occurs in the first 30 to 60 minutes. Reservoirs can be built into the internal surface of the tray (Figure 14-15) on the buccals of some or all of the teeth to increase the speed of the bleaching by leaving more carbamide peroxide in contact with the dental surfaces. The tray is generally quite thin and is made of transparent material so it can be worn during the day, even during work (Figure 14-16).
Disadvantages of the tray system include the need to acquire an impression (Figure 14-17) of the dentition prior to tray fabrication. This impression is then poured in stone, and a tray is fabricated with a heat and suck-down tray-former such as the UltraVac Vacuum Former (Figure 14-18) (Ultradent Products, Inc.). This device is relatively easy to operate but does require some chairside and in-office laboratory time. Typically tray fabrication in the dental office can be delegated to an auxiliary who will complete the task in 30 to 60 minutes. Since the bleaching treatment is often an impulse decision for the patient, it is typically unplanned. As a direct result, tray fabrication can contribute to scheduling problems. However, it is important once the patient decides to have their teeth bleached to begin treatment as soon as possible, thus taking advantage of the patient’s active, but possibly fleeting, interest in the color of their dentition. Furthermore, most auxiliaries are not particularly fond of trimming the bleaching trays (Figures 14-19 and 14-20), which should terminate just shy of the gingival margin of the soft tissues. Generally it is a good idea to not have the bleaching tray impinge on the soft tissues (Figure 14-21), as this may cause gingival irritation and patient discomfort. The tissues must be approached as closely as possible (Figure 14-22) to maximize the whitening effect and to minimize treatment time. Customizing the margins to adapt them to the dentition takes several minutes and can be exacting, even with the proper scissors.
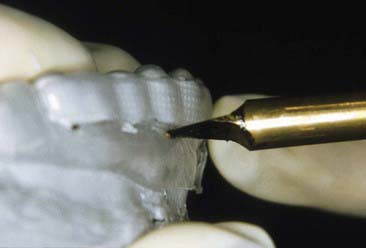
FIGURE 14-20 Custom bleaching trays should be trimmed just shy of the gingival margin of the soft tissues.
Prefabricated Tray and Tray-less Bleaching Systems
Disadvantages include: the inner, soft tray material (Figures 14-23 and 14-24) that is adapted to the teeth can slip off sooner than desired, leaving the teeth less bleached than anticipated. Most patients lose comfort (and patience) with the bleaching material on their teeth after about 20 or 30 minutes, and sometimes even less. The soft tray is very easy to remove. Thus compliance with the prefabricated tray system is not as predictable as with the custom-fabricated tray systems.

FIGURE 14-23 The soft tray material in the tray-less bleaching systems may slip off sooner than desired.
Strip systems can be limited by their overall length; they cover the teeth cuspid to cuspid but not much beyond these teeth (Figure 14-25). Once strip bleaching is completed, the cuspids or first bicuspids will be whitened but the molars will remain more or less at their original color. If these teeth are visible on the smile, supplemental tooth whitening treatment is necessary.
At-Home Bleaching Considerations
The Safety of Tooth Whitening
1. A new product can be tested on animals in order to predict its toxicity in humans
2. The performance record of the product or a material that has previously been used for human treatment can be examined for deleterious side effects.
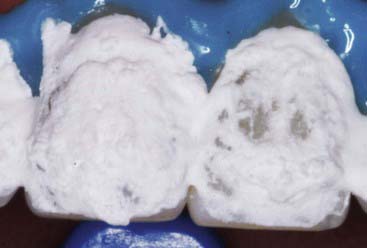
Carbamide peroxide is not a substance that is new to dentistry, nor was its development for dental purposes accidental. Aqueous hydrogen peroxide has long been used by the dental (and medical) profession; its lack of toxicity and minimal side effects, combined with both cleansing and bactericidal properties, make it particularly attractive for intra-oral use. A major practical problem with hydrogen peroxide is its extremely rapid breakdown on contact with body tissues, a reaction that is greatly accelerated by peroxidase and catalase enzymes, which are commonly found in the mouth and the body. Foaming (Figure 14-26) is often observed at the initial application (or re-application) of hydrogen peroxide whitening agents. This is an oxygenated foam that demonstrates the catalysis of the peroxide. A 10% preparation of carbamide peroxide in anhydrous glycerin is equivalent in chemical activity to 3% aqueous hydrogen peroxide, yet far more stable and predictable.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses