Chapter 14
Autogenous Block Graft
Onlay grafting is a method in which the graft material is placed over the defective area to increase width and/or height of the alveolar jawbone when there is insufficient volume to accommodate endosseous implants, hence the need for bone reconstructive treatment (Collins 1991). This method can also be used to level deformities in the bone contour or to cover dehiscence. In minor graft procedures, small pieces of bone may be harvested from the chin or retro-molar area or tuberosity (intraoral source), and the host bed is usually perforated by means of a small round bur to stimulate the formation of a blood clot between graft and recipient bed. The graft is immobilized with screws and plates or with dental implants (Kahnberg, 1989; Kahnberg, Nystrom and Bartholdsson 2005). In major graft procedures, bone from the iliac crest or calvaria (extraoral source) is most commonly used to repair defects due to severe bone deficiency.
All these grafts are called “autogenous” (or “autografts”), because the missing bone is replaced with bone from the patient’s own body, that is, by transplanting the bone tissue from one site to another (recipient site) within the same individual. The sources of autogenous bone grafts may be either intraoral or extraoral. The two main intraoral sources of block grafts are the mandibular symphysis and the mandibular ramus. As already mentioned, one extraoral source of block grafts include the iliac crest; but due to its endochondral origin, it has been shown to have increased resorptive properties (Zins 1983), whereas the resorption observed for mandibular sites seems to be lower. In addition, with the iliac crest harvesting procedure, an increase in morbidity is observed, associated with the need for hospitalization (Shwartz-Arad 2005). Implant reconstruction does not require the harvesting of as large a bone volume as that harvested from the iliac crest; therefore, this latter donor site is not indicated other than for large reconstructive surgeries. Moreover, recent advances in piezoelectric surgery (PS) have allowed easier harvesting from the mandibular ramus and symphysis, which in turn facilitates the procurement of even larger-size bone grafts because the piezoelectric cutting tip allows a more precise bone-saving cut (Vercellotti et al. 2005).
After a thorough exploration of the morphology of the bony defect to select a suitable bone-promoting technique, most commonly an autogenous bone graft is chosen. Indeed, autografts are considered the gold standard in bone grafting since they are essentially living tissues with their cells intact. Thus, no immune reaction is to be expected, and the outcome—especially in terms of healing and prognostic predictability—is very often successful as compared to other techniques using alloplastic and allogenic materials. Also, autogenous block grafts have superior biological and mechanical properties, and—being autogenous in nature (i.e., osteoconductive and osteoinductive)—represent an ideal choice for grafting large defects, ranging from Seibert class I to Seibert class III defects.
Importantly, all types of autografts have similar regenerative processes, but the success of the grafting procedure basically depends from the quality and intensity of revascularization(Burchardt 1983). In the absence of blood supply, the bone tissue dies and the bone collapses. However, besides the quality of the donor site, the graft revascularization also depends on the regenerative potential of the recipient site, which is generally unknown prior to surgery. Thus it is necessary to resolve the problem of improving graft regeneration and revascularization while maintaining bone density and osseointegration properties. In this regard, it is worth mentioning some theories addressing this issue, such as, for example, the so-called osteoblast theory by Wolff in 1863 (Glicenstein 2000), according to which grafted bone healing occurs by osteogenesis. In 1893, Barth introduced the “framework theory,” stating that regeneration takes place through osteoconduction (colonization of the mineral part by osteoblasts from the recipient site). Today, studies mainly support the osteoinduction principle (osteoinduction implies the recruitment of immature cells and their stimulation to develop into preosteoblasts, thus inducing osteogenesis) for regeneration (Boyne, 1997), but all three confirm the graft of autogenous bone as the most advantageous in terms of regenerative capacity.
ADVANTAGES OF CHIN BONE HARVESTING
The chin, or menton, the lowest point of the mandibular symphysis, has been reported in the literature as an important donor site in preprosthetic reconstructions because the bone in this region is usually dense (mean volume obtained from a symphyseal harvest, about 4.71 mL). Multiple advantages of chin bone harvesting have been documented in the literature (Reddi 1987; Pikos 2005a, 2005b). As previously stated, onlay grafts are used especially when bone height or width are an issue, and chin block grafts (self-donated chin bone) are often successfully used to reconstruct the ridge defect before implant placement. The areas of the chin bone from which bone is extracted are known to regenerate very quickly and to provide what is considered to be the healthiest and best source of augmentation material, with only minimal complications and significantly less resorption. In addition, intramembranous bone grafts maintain more volume than endochondral ones, possibly due to their faster revascularization. Indeed, the symphyseal region can provide higher quantities of bone compared with other intraoral sites. However, in order to avoid excessive resorption of the chin graft, it is recommended that fixture installation be performed within 4 months from the graft placement, except in particular cases (Sindet-Pedersen and Enemark 1990; Pikos 1995).
Moreover, another main advantage of chin bone harvesting is the ease of access to the harvest site, with enhanced proximity to the donor site. The bone harvested from the symphysis is an embryonic one, which means that it is vascularized quickly, and this leads to less resorption, making chin bone a good graft material for the preparation of the implant bed. It has been documented that the highest concentrations of promoter proteins, such as bone morphogenetic protein (BMP) or osteogenin, are found in the cortical structure (Urist, Mikulski and Lietze 1979; Urist and McLean 1952).
Furthermore, because harvesting of chin grafts is generally done under local anesthesia, after local infiltration and blocking of both mental nerves to reduce intraoperative bleeding, there is no need for hospitalization, which, taken together with minimal morbidity, a short healing period, and absence of cutaneous scars, represents an attractive option for the patient.
Chin grafts may be useful in managing both maxillary and mandibular reconstruction procedures, especially when small- to medium-size blocks are needed (Pikos 1996).
ADVANTAGES OF RAMUS BONE HARVESTING OVER SYMPHYSIS GRAFT (MISCH 2000; CAPELLI 2003; TOSCANO ET AL. 2010)
The mandibular ramus block graft can provide sufficient bone for medium- to long-span reconstructions, with augmentation of 3 to 4 mm being achieved both in the horizontal and vertical directions (Pikos 2005a, 2005b), In fact, ramus grafts are indicated in moderate to severe localized defects, for example, in one- to four-tooth edentulous spans. If the third molars have been extracted or congenital missing of teeth is observed, or if there is not enough bone to harvest from the chin area, the ramus site can be used. In addition, a bilateral harvest from both rami gives the option to harvest a larger volume of bone when reconstructing a large edentulous area. Thanks to the thickness of the ramus cortical bone blocks, it is possible to obtain rectangular grafts of appreciable size, although some trouble exists in accessing this donor site. However, when harvesting bone from the mentum, there is a risk of altered facial contour, which is why the donor area should be grafted with particulate bone after harvest; this is not a concern when harvesting from the ramus. Moreover, the proximity of the ramus to the mandibular recipient sites makes it an excellent donor source. Mostly cortical bone is harvested from the ascending ramus of the mandible; with these grafts, a lower incidence of complications is observed than during chin graft procedures. The advantages of this donor site over the chin also include a decrease in complaints of postoperative sensory disturbances of the face and teeth by the patient and absence of aesthetic concerns (Pikos 1996; Misch and Misch 1995). Overall, fewer complications are reported with ramus bone harvesting than with the chin, and resorption rates are more than acceptable. Furthermore, long-term morbidity associated with mandibular ramus bone grafts is reported to be slightly lower than with symphyseal bone grafts. As already mentioned above, however, the surgical access is somewhat limited, and in a few cases there might be some damage to the mandibular neurovascular bundle. In any case, it appears that more ramus grafts are performed than chin blocks. Actually, the use of the mandibular ramus as a donor site is often preferable because of associated lower morbidity and postoperative pain. Symphyseal bone grafts offer bone quantity and quality, and chin ptosis is not a concern, as long as the inferior border is not exceeded and the mentalis muscle is managed well. Nevertheless, the ramus graft requires a short healing period (4 to 5 months) and shows minimal bone resorption while providing a high-density bone quality (Capelli, 2003).
More generally speaking, it is also important to note that classical studies, as well as several reviews conducted on both ramus and symphysis grafts, report a range of about 1- to 7-mm, with an average 4-mm, augmentation width increase. There are also advantages of blocks in terms of better bone quality for implants and faster integration (implants can be placed in 4 months vs. 6–9 months, compared with guided bone regneration). However, there is a lack of long-term studies on blocks. It is known that osteocytes in the block do not survive, and the block is turned over by gradual penetration of osteogenic tissue into the site to form new bone (creeping substitution). Resorption is known to be about 25% between the time of the block graft and the implant placement, but it would be important to know how much bone is resorbed and reconstructed over time. No such reports, however, are yet available in the literature (Toscano et al. 2010).
The rationale for using barrier membranes is the prevention of graft resorption, although the reviews of the efficacy of barrier membranes have been complicated by the inclusion of uncontrolled studies. Despite that, the current best evidence would suggest that it is reasonable to state that membranes show some preventative effects on graft resorption.(Rasmusson et al. 1999, Gielkins et al. 2007)
Ramus grafts are contraindicated in the following cases: the width of the ramus is less than 10 mm; the mandibular canal is positioned superiorly; the patient has limited jaw mobility or has previously undergone a sagittal split osteotomy; and the presence of third molar pathology may also potentially affect the harvested bone. Also, it is important to remember that thickness and morphology of the bone grafts harvested from the ascending ramus are not homogeneous and that, from a qualitative point of view, they are monocortical, with little or no cancellous bone.
Both the donor sites considered share a common embryological origin (first and second brachial arches) and, being membranous, have slow resorption rates.
SURGICAL TECHNIQUES
Chin Graft
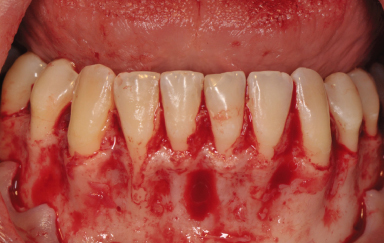
The incision design is either intrasulcular or vestibular. The intrasulcular design is contraindicated in the presence of healthy periodontium, crown and bridge work, and thin biotype (Pikos 1996; Figure 14.1). Similar to any other flap procedure, some crestal bone loss can be expected with this procedure. In addition, dehiscences and fenestrations could be exposed by this technique, and if the remaining bone is thin, surgical trauma and loss of periosteal blood supply to one-third of the facial plate could induce irreversible bone loss at the crest, followed by soft tissue recession. Repositioning of the tissue is difficult, especially when the mentalis muscle is stripped from its attachment. Usually, the intrasulcular incision is made on the facial aspect of the lower anteriors as far distal as the canines, with a full-thickness flap to expose the anterior surface of the mentum (Figure 14.2).
Figure 14.1 Sulcular incision.
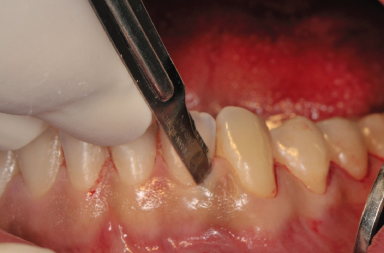
Figure 14.2 Exposure of the chin donor area after sulcular incision.
The vestibular incision is made as a shallow incision at the bottom of the vestibule, between the first premolars and in continuity with the bilateral crestal incisions to give access to the symphyseal region, thus allowing the design of the grafts needed. Mucosa, muscle, and periosteum are cut in a through-and-through fashion. After the first vestibular incision, the blade is turned perpendicular to the periosteum and the attachment of the mentalis muscle is incised to allow later suturing of the loose muscle to the part remaining attached to the bone. A full-thickness flap is reflected to expose the donor site (Figures 14.3, 14.4). The advantages with this approach are that it does not interfere with the periodontium of the lower anterior teeth, t/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


