14
Acute and Chronic Infections Affecting the Oral Cavity: Orthodontic Implications
Summary
Infectious diseases occur worldwide, posing multilevel challenges to orthodontists everywhere. Each new patient must be examined thoroughly to uncover the possible presence of infections that could be potentially dangerous for others. Recognition of pathological conditions in a patient’s orofacial region before or during treatment requires a precise diagnostic technique to eliminate all potential health risks to other patients and staff in the orthodontic clinic. In those cases where patients have such infections, it is advisable to promptly interact with pertinent medical specialists to obtain confirmation of the disease identity, its treatment, and any bearing of the disease and medications on the orthodontic treatment plan and projected outcome. Both infectious diseases and the medications taken for their treatment may affect the tissue remodeling response to orthodontic forces. This chapter discusses the features of infectious diseases, both acute and chronic, that may profoundly affect the diagnosis, course, and outcome of orthodontic treatment. These infectious diseases are caused by bacteria, viruses, fungi, and parasites and some may be potentially lethal. The goal of this chapter is to stress the importance of identifying these diseases during the diagnostic phase of orthodontic therapy and of seeking advice from medical specialists to prevent the spread of the diseases and achieving optimal orthodontic outcomes.
Introduction
Orthodontics, a specialty based on solid scientific foundations, is being practiced predominantly as a mechanical art, due, at least in part, to its close association with dentofacial esthetic dogmas. Orthodontists are keen to correct facial and dental disfigurements, but often little attention is paid to the general health status of patients, as if the dentofacial region exists in isolation from the rest of the body. This approach can be termed as ‘regional diagnosis’, where an emphasis is placed on the face and oral cavity while ignoring the overwhelming evidence on an intimate association between dentofacial events and a multitude of pathophysiological developments throughout the rest of the body (Moore, 1939). Such a narrow approach to orthodontic diagnosis and treatment planning, which overlooks basic biological principles, may not only jeopardize treatment outcomes but also the wellbeing of the patients. Thus it is essential to shift the paradigm of orthodontic patient evaluation to encompassing all aspects, both medical and dental, in the diagnostic datasheet, with due consideration of all health-related issues.
Orthodontic tooth movement results from profound remodeling activities of all dental and paradental tissues in response to the application of mechanical forces to the crowns of the teeth. These remodeling activities are performed by various cells, including cells of the nervous, immune, endocrine, and skeletal systems, providing tooth movement with a noticeable systemic involvement. Therefore, orthodontics should be considered as an integral part of medicine, and aiming to treat and correct malocclusions while weighing the possible effects of each patient’s systemic condition on his/her orthodontic diagnosis and treatment plan. This definition introduces biological factors into orthodontic practice, and increases the probability of attaining pleasing and durable results (Childs, 1933).
Orthodontists often see patients with acute or chronic infectious diseases or with adverse reactions to the mechanotherapeutics they practice. It is helpful to refer these patients to competent physicians for advice that will enable the orthodontist to expand the boundaries of the diagnostic process, and craft detailed individual treatment plans. Creation of such a communication bridge will augment the union between orthodontics and medicine to the benefit of all individuals in need of orthodontic care (Dunning, 1941).
The close relationship that an orthodontist should maintain with other medical and dental specialists depends on the extent of his or her knowledge base, on which depends the ability to identify conditions requiring and/or justifying patient referral to appropriate medical professionals (Weinberger, 1938). An orthodontic practice might encounter patients with oral manifestations of a variety of diseases. Recognizing these signs and symptoms should prompt the practitioner to perform specific laboratory tests to facilitate making the correct diagnosis, identifying the course of the disease process, and assessing its effects on the treatment plan and the course of treatment. Such a routine could include differentiation between infectious and noninfectious diseases, and aid in deciding whether orthodontic treatment can be provided without further delay, or if a referral to a medical specialist for consultation is deemed necessary prior to formulating a comprehensive diagnosis. Lack of adequate knowledge of infectious diseases can cause unnecessary delays in instituting appropriate therapy, and at the same time lead to failure in implementing proper safety measures for prevention of disease transmission to the orthodontic office personnel. This chapter will concentrate on non-odontogenic infections and their possible orthodontic implications (odontogenic infections are discussed in subsequent chapters of this book). Systemic infectious diseases with oral manifestations and with a high degree of microbiological specificity are discussed, providing a clear guide to distinguish between bacterial, viral, fungal and parasitic infections.
In many systemic infectious diseases, the salient causative organisms have been identified and characterized as bacteria, viruses, fungi, or parasites. The infectious nature of these diseases requires the implementation of strict hygienic procedures and disinfection, to avoid any chance of contamination and transmission of the disease in the orthodontic office. To achieve this goal, it is imperative to identify at the first appointment itself, patients who may be carriers of such diseases, prior to proceeding with any clinical activities. However, in addition to the efforts to eliminate the risk of spreading an infectious disease to other people in the orthodontic office, orthodontists should realize that infectious diseases, with no exception, modify the immune system of each infected person. Such modifications may have profound effects on the paradental cellular and tissue response to orthodontic forces. Inflammation is a major part of this response, intimately involved in every step of the tissue remodeling process that facilitates tooth movement. Signaling molecules produced by migratory primed inflammatory cells reaching the mechanically stressed periodontal ligament (PDL) are capable of both accelerating and inhibiting the rate of tooth movement, depending on the nature of the specific molecules involved. Moreover, patients who have been diagnosed as having acute or chronic infectious diseases are usually being treated with medications that may markedly alter the course and outcome of orthodontic therapy, primarily by affecting the patient’s immune system and the paradental inflammatory process. All these facets of infectious diseases dictate that a consultation with a physician specializing in treating patients with these diseases is imperative prior to the onset of any orthodontic treatment. This communication between the orthodontist and the physician must continue throughout the course of treatment in the event of any change in the health status of the infected patient during this period.
Bacterial Infections
The resident and transient microflora of the oral cavity consists of more than 700 bacterial species or phylotypes, providing a useful defense mechanism against the establishment of more pathogenic species (Aas et al., 2005). The resident oral flora can be divided into the indigenous flora, that is, those are found in almost all humans irrespective of environmental conditions (e.g. species of Streptococcus, Actinomyces, Haemophilus, Neisseria, Fusobacterium, and Prevotella), and the supplemental flora, which have specific requirements for adhesion, nutrition, redox potential, and pH levels (Dahlèn, 2009). The species of transient flora do not permanently colonize the oral cavity, have low virulence and live in harmony with the hosts. This might include some opportunistic pathogens, such as Staphylococcus aureus, pneumococci, enterococci, enteric rods, and yeasts. If the host’s immunity becomes impaired, these bacteria might initiate an infectious process (Kononen, 2000). Moreover , impairment of the host immune response destroys the microbial homeostasis, which allows some pathogens to grow in greater numbers and cause an infection characterized by increased pathogen load and inflammation.
Mucosal Lesions of Uncertain Microbiological Basis
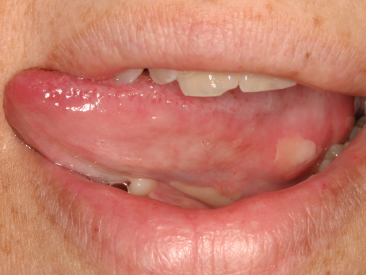
Mucositis
Mucositis is a form of mucosal barrier injury, characterized clinically by oral erythema, ulceration, and pain (Figure 14.1). It is a common complication of therapeutic procedures involving chemotherapy, radiotherapy, or both, and in patients receiving bone marrow transplants, which are damaging to the epithelial cells (Scully et al., 2003). The role of microorganisms in mucositis is uncertain as regards whether they have a role in the initiation of the lesion, or whether they secondarily infect the damaged area or the ulcer. Commonly, the oral manifestation of cancer therapy is a generalized stomatitis whose association with the primary underlying infection is more obvious (For further details, see ‘Oral mucosal bacterial infections’, p. 243). Secondary bacterial infections may also arise in bite wounds, and in viral stomatitis and aphthous ulcerations, and lead to streptococcal bacteraemia in any area of the mouth, such as the tongue, buccal mucosa, floor of the mouth, palate, and gingiva.
In addition, there is increased occurrence of opportunistic pathogens such as aerobic Gram-negative bacilli (AGNB) including enterics (Escherichia coli, Enterobacter spp.) and pseudomonads, as well as yeasts (Candida spp), in mucositic lesions. If xerostomia prevails, oral health can be seriously compromised (Sonis, 2007). Xerostomia can lead to decreased clearance of oral microorganisms, along with decreased pH, resulting in increased colonization by lactobacilli, Candida albicans, and other opportunistic microorganisms, such as Staphylococcus aureus, enterococci, and enteric rods (Pajukoski et al., 2001).
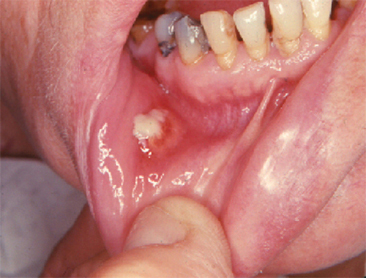
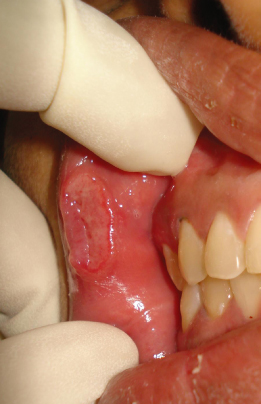
Recurrent Aphthous Stomatitis
Otherwise known as canker sore, this is the most common nontraumatic type of oral ulcer (Figure 14.2). The sore is often mistaken for a virus infection (herpes simplex), and is differentiated by appearance only in the nonkeratinized mucosa, such as the labial mucosa, buccal mucosa, ventral tongue, and vestibule (Ship et al., 2000). Based on their clinical appearance, aphthous ulcers can be classified as minor (<1 cm in diameter), major (>1 cm diameter), and herpetiform, in which multiple minute ulcers and which might coalesce to form plaques (Woo and Sonis, 1996). Most aphthae are of the minor variety, and heal within 10 days. Factors predisposing to this condition include stress, poor nutrition, infection, hormonal fluctuation, and trauma (McCullough et al., 2007). The disease is usually self–limiting, and nutritional supplementation, alleviation of stress, and warm saline rinses have been found to help in its management. Orthodontic attachments are considered to be one among the main reasons for triggering their appearance. If the ulcers appear following orthodontic bonding, the irritating attachment can be either covered with orthodontic wax or removed to prevent further irritation aggravating the process.
Figure 14.2 Major aphthous ulceration.
Burning Mouth Syndrome
Scala et al. (2003) defined the burning mouth syndrome as a chronic pain syndrome that mainly affects middle-aged/older women with hormonal changes or psychological disorders. This disorder reduces the quality of life, especially with large psychological implications. Although the etiology of this syndrome remains unclear, the presence of salivary gland dysfunction or systemic diseases, such as diabetes, makes a patient prone to it. In addition, patients with chronic pulmonary diseases, asthma, and rheumatic arthritis can develop epithelial atrophia, glossitis, xerostomia, and the burning mouth syndrome. All these symptoms are due to superimposing fungal and bacterial infections that follow adverse reactions to immunosuppressive medications and reduced salivary flow. Chemotherapeutic agents also have the potential to induce changes in the oral epithelium, leading to superinfection (Lopez-Jornet et al., 2010).
Oral Mucosal Bacterial Infections
Acute mucosal infections of the oral cavity may appear locally, or as generalized stomatitis, and can be asymptomatic or accompanied by mild discomfort or severe pain (Dahlèn, 2009). Patients often neglect these conditions, unless they become bothersome. The most common symptoms are burning sensations, followed by clinical signs of redness and epithelial atrophy or desquamation. Helovuo et al. (1993) observed an increased prevalence of E. coli, Enterobacter spp, and Staph. aureus after treatment with penicillin and erythromycin. Patients with conditions causing immunocompromise, such as leukemia and other malignancies, are at high risk of developing oral mucosal ulcerations due to drug consumption and development of granulocytopenia. Similar conditions have been found in patients with organ transplants and rheumatic arthritis (Dahlèn, 2009). As a result of decreasing general health and increased medication intake, older people are more prone to develop oral mucosal lesions (Sweeney et al., 1994). Dahlèn et al. (2009) recently confirmed that bacterial mucosal infections are more prevalent than fungal infections in the oral cavity. They also observed a marked decline in the levels of normal mucosal bacteria, such as viridians streptococci (α-streptococci), Neisseria, and Haemophilus spp.
Dentists, more specifically orthodontists, frequently ignore such conditions, and/or leave them underdiagnosed. Infections associated with the oral cavity have a unique microbiological base, often a mixed one, making it difficult to identify a specific, salient microorganism. The best diagnostic approach starts with comprehensive history taking, which should be followed by a thorough physical examination. Microbiological laboratory investigations and antibiotic susceptibility tests should be conducted routinely on orthodontic patients with oral lesions, so that the conditions can be effectively and properly managed. An orthodontist accepting patients in this category should emphasize the importance of maintaining impeccable oral hygiene to postpone or minimize the development of oral mucosal ulcerations. It is also highly recommended to refer any patient with an oral microbiological infection that might be contagious, for a consultation to a physician. The infections arising in the oral cavity are listed in Table 14.1.
Table 14.1 Bacterial infections of the oral cavity
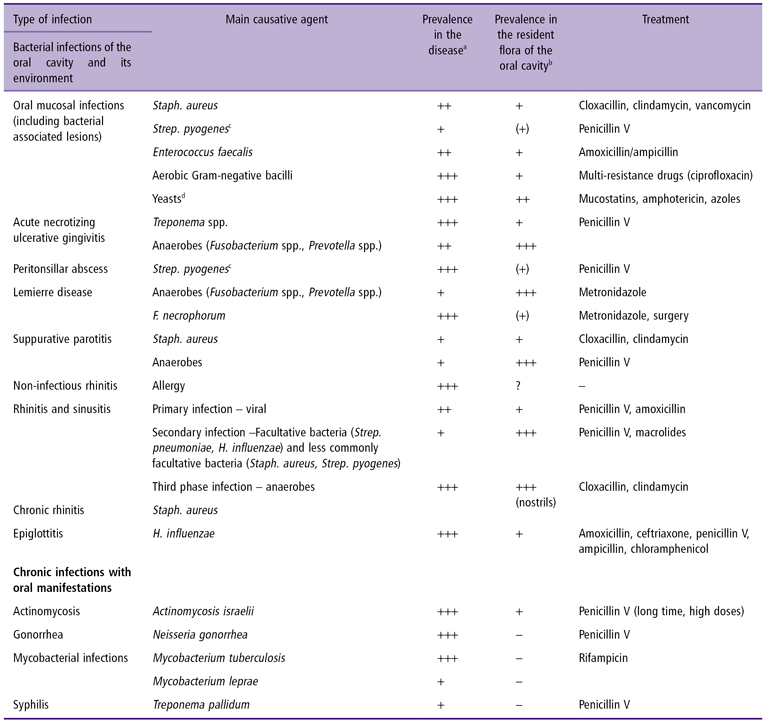
aPrevalence: + <10%, ++ <30%, +++ >30%.
bNot isolated, (+) sporadically present in low numbers, + present in <10% and in low numbers, ++ commonly present <30% sparsely or in moderately heavy growth, +++ usually present and in the predominant flora.
cGroup A hemolytic streptococci.
dCandida spp.
Aerobic Infections of the Oral Cavity
Microorganisms causing oral mucosal infection have a predominantly aerobic character, and in the majority comprise staphylococci, enterococci, AGNB, and yeasts. They are all categorized as classical opportunists, and are commonly associated with immunocompromise (Dahlèn et al., 2009), although they have been noted in healthy individuals. These aerobic infections are difficult to treat due to a pronounced resistance against common antibiotics by these microorganisms and a low response as long as the compromised condition prevails. A microbiological diagnosis is strongly recommended since the treatment strategy may be quite different, depending on the identity of the involved microorganisms. Treatment of these infections is also sometimes challenging due to a combination of infections caused by two or more of these opportunists. A brief description of bacteria commonly producing oral mucosal infections is provided below. Yeasts involved in the process are discussed on page 256–259.
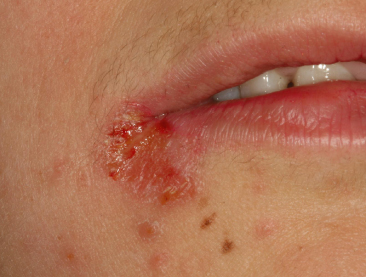
Staphylococcus Aureus
Staph. aureus is considered to be a relevant pathogen in symptomatic oral mucosal lesions. Until then, if isolated, it was considered to be a part of a transient microbial flora that seems to increase with advanced age. This bacterium is frequently isolated from infections of the facial skin and lips (Faergemann and Dahlèn, 2009). Angular cheilitis is commonly secondarily infected by Staph. aureus (Figure 14.3), with a particular predilection in young individuals and those with dry skin. If a healthy individual harbors high numbers of salivary Staph. aureus (>100 000 colony-forming units/mL) over an extended period of time, they are considered to be healthy carriers (Dahlèn, 2009). Infected medical and dental professionals pose a risk of transmitting it to other individuals visiting them. A serious concern in this regard is the infection caused by methicillin-resistant Staph. aureus. Treatment of infection with Staph. aureus typically included penicillinase-stable penicillins, such as cloxacillin, dicloxacillin, or flucloxacillin, because of the risk of developing resistant strains (Dahlèn, 2009).
Enterococci
Enterococci, particularly Enterococcus faecalis, are commonly involved in oral mucosal infections. These infections occur sporadically in the oral mucosa and around the teeth in healthy individuals but enterococci are a typical opportunist in immunocompromised patients, often in combination with other opportunists such as enterics and yeasts (Dahlén, 2009). Enterococci are of particular interest to dentists due to their persistence even after treatment of endodontically involved teeth.
Enterococci were known as streptococci for a long time, and were classified as Lancefield group D. However, owing to their survival in harsh environments (temperature, dryness, salts, dyes, antiseptics, and antibiotics), they were classified as a new genus, which normally resides in a significant part of the upper intestine and is resistant to bile salts. It is spread to the oral cavity by vomiting and faecal contamination. Enterococci are commonly used in the food processing, e. g. cheese, and spread through food cannot be excluded (Templer et al., 2008). It is resistant to penicillin V and clindamycin, and broad-spectrum penicillins such as ampicillin and amoxicillin remain the drugs of choice (Dahlén et al., 2009).
AGNB, including E. coli, Enterobacter spp., Klebsiella spp., Proteus spp. and more, are typically involved in human opportunistic infections, including oral mucosal infections. AGNB are part of the normal resident intestinal flora, frequent participants in nosocomial infections, and cause the classic opportunistic infections affecting the oral cavity. Transmission of these bacteria to the oral cavity can occur through poor personal hygiene, or through food and drinking water. They are frequently associated with periodontitis and peri-implantitis. They rapidly become drug-resistant, and the drug to be used should be selected only after a susceptibility test, although the choices are more or less exhausted. Quinolones (ciprofloxacin, norfloxacin) are the drugs of choice for the infections with AGNB, but should be prescribed only under proper medical supervision. Empirically, it has been observed that it is difficult to harm the bacteria and for the infection to heal as long as the patient is in a compromised state (Dahlèn et al., 2009).
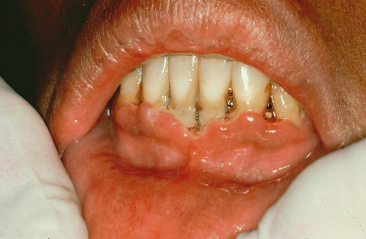
Acute Necrotizing Ulcerative Gingivitis
Acute necrotizing ulcerative gingivitis (ANUG) is an infectious disease of the gingiva (Figure 14.4), caused by a mixed flora consisting of spirochetes, fusobacteria, Prevotella intermedia, Veillonella species, and streptococci (Rivera-Hidalgo and Stanford, 1999). This disease often results in gingival bleeding, ulceration, and episodes of severe pain. Factors predisposing to this condition include stress, smoking, malnutrition, and poor oral hygiene (Horning and Cohen, 1995). ANUG is common in patients with human immunodeficiency virus (HIV) infection. If it is associated with rapid alveolar bone loss, it is categorized as necrotizing ulcerative periodontitis, in which severe deep aching pain is a characteristic feature (Murayama et al., 1994). The treatment of this condition is directed towards debridement of affected soft tissues and teeth, along with antibiotic therapy (Rivera-Hidalgo and Stanford, 1999). A referral to a competent periodontist, if an orthodontist encounters such a condition, can be of great value in its management.
Acute Pharyngitis
Acute pharyngitis, otherwise known as a sore throat, can have either viral or bacterial etiology. The most common bacterium involved is Streptococcus pyogenes (β-hemolytic streptococci or group A streptococci [GAS]) (Peterson and Thomson, 1999). During the acute phase of the disease, these microorganisms can be detected in the saliva. This disease is among the 20 most common diseases that occur without any age, sex, or racial predilection. The exotoxins produced in this infection by the β-hemolytic streptococci act as superantigens that up-regulate the T lymphocytes, prompting the release of proinflammatory cytotoxins, and have synergistic effects along with the lipopolysaccharides of the extracellular matrix. The superantigens then evade the pharyngeal immune response, resulting in proliferation of Strep. pyogenes. The most common symptoms of acute pharyngitis are sore throat, odynophagia, headache, nausea, vomiting, and abdominal pain. On physical examination, the patient may have fever, tonsillopharyngeal erythema, exudates, beefy red swollen uvula, anterior cervical tender lymph nodes, petechiae on the palate, and scarlatiniform rash. The gold standard diagnostic test for acute pharyngitis is throat culture, but unfortunately this test takes 24–48 hours to be completed (Halsey, 2009). Instead, rapid antigen detection tests (RADTs) with commercial kits are available but these are relatively expensive. Samples for throat culture or RADTs should be obtained from the posterior pharynx or the tonsils. Imaging studies have no role in the diagnosis of this disease.
Oral penicillin V is the drug of choice for this disease. Amoxicillin remains a better alternative, but tetracyclines or sulfamethoxazole should not be used due to the high resistance rate. In the case of penicillin allergy, cephalosporins, which contain a β-lactam ring, can be used with caution. In rare cases, the pharyngitis spreads to adjacent areas, forming abscesses requiring surgical drainage. Surgery is an option, especially if the history reveals recurrent tonsillitis (Brook, 2009). The patient should be allowed to eat a normal diet and drink warm liquids that provide symptomatic relief (Halsey, 2009). An otorhinolaryngologist should be consulted for local suppurative complications such as peritonsillar abscess and mastoiditis. An infectious disease expert should be consulted for patients with immunocompromising conditions, or when HIV infection is suspected.
A rare but often underdiagnosed form of an oropharyngeal infection is the Lemierre syndrome, an infection caused by Fusobacterium necrophorum (Karkos et al., 2009), which occurs in adolescents and young adults as a complication of tonsillitis (‘sore throat’), especially in recurrent forms (Klug et al., 2009). It is life-threatening if neglected, and should be treated aggressively with antibiotics (metronidazole) and/or surgery (Ridgway et al., 2010).
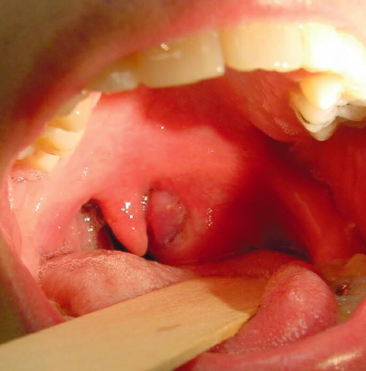
Peritonsillar Abscess
Peritonsillar abscess (PTA) is a localized accumulation of pus in the peritonsillar tissues, which forms as a result of suppurative tonsillitis (Figure 14.5). The peritonsillar space is bounded by the tonsillar pillars anteroposteriorly, piriform fossa inferiorly, and the hard palate superiorly. The area can be infected with both aerobic (S. pyogenes) and anaerobic (Prevotella and Peptostreptococcus spp.) bacteria. The infection begins superficially, progresses into deep tissues and has no age, sex, or racial predilection. The symptoms include sore throat, dysphagia, and change in the voice, headache, malaise, fever, neck pain, otalgia, and odynophagia. Physical examination reveals mild to moderate discomfort, fever, tachycardia, dehydration, drooling of saliva, trismus, cervical lymphadenitis, asymmetric tonsillar hypertrophy, inferior and medial displacement of a tonsil, contralateral deviation of the uvula, and erythema and exudates from the tonsils. No investigations are required to diagnose PTA but if a need is assumed, needle aspiration followed by culture of the fluid obtained can be performed (Peterson and Thomson, 1999; Mehta et al., 2009). Patients are often managed in the outpatient department, unless they show signs of airway obstruction, sepsis, toxicity, or other complications. Along with incision and drainage, analgesics, and throat wash, antibiotics are often prescribed for complete resolution of PTA. A combination therapy of penicillin and metronidazole is often preferred. Clindamycin is a good choice in people with penicillin allergy. Consultation with an otorhinolaryngologist should be sought whenever a PTA is recognized, before, during, or after the onset of orthodontic treatment, for proper management of these patients. Help from an anesthetist might sometimes be required if the patient develops difficulty in maintaining airway patency.
Figure 14.5 Peritonsillar abscess.
Suppurative Parotitis/Sialadenitis
Acute suppurative parotitis (ASP) is an infectious process of the parotid gland, usually seen in elderly people who are dehydrated, malnourished, or recovering from surgery (Raad et al., 1990). Although the infection is usually confined to the parotid capsule, it can occasionally spread to cervical fascial planes. Immunosuppression states such as diabetes, alcoholism, HIV infection, autoimmune disorders (e.g. Sjögren’s syndrome), poor oral hygiene, decreased salivary flow, postsurgical dehydration, sialolithiasis, tumors or foreign bodies in the duct, can increase the risk for ASP. The most common clinical manifestation of ASP is an indurated, erythematous, warm swelling of the cheek. The patient will also have mild fever along with pain. Intraorally, the orifice of Stensen’s duct will be red, and pus may be expelled while palpating it (Brook, 2009). In rare instances, facial nerve dysfunction may be the end result (Noorizan et al., 2009). Diagnosis can be established by a thorough clinical examination, along with a complete blood count and chemistry, and culture and sensitivity. Radiological evaluation should include CT scanning with an intravenous contrast medium, ultrasound scanning for detection of abscesses and also sialography.
Traditionally, the main causative agent of ASP is thought to be Staph. aureus. However, Brook et al. (1991) have isolated strict anaerobes such as Peptostreptococcus spp. and Gram-negative anaerobic rods from ASP lesions. The key treatment for ASP is rehydration and supportive treatment, which should include intravenous fluids, nutritional support, a warm compress, sialogogs, good oral hygiene, and antibiotic therapy. The drugs of choice in ASP are penicillin, first-generation cephalosporins or clindamycin, however, more important is the selection of the drug in relation to the causative agent. Intraductal injections of antibiotics usually lead to improvement in the condition. If there is lack of improvement after 3–5 days of antibiotic treatment, and if there is facial nerve involvement or involvement of adjacent vital structures, surgical opinion must be sought; usually incision and drainage via a standard parotidectomy incision will be the preferred treatment. Proper follow-up, repeated clinical examinations, fine needle biopsy, and imaging should be performed on a regular basis to ensure the absence of any neoplastic changes in the gland (Brook, 2009).
Rhinitis and Sinusitis
Rhinitis implies a heterogeneous group of nasal disorders, characterized by sneezing, nasal itching, rhinorrhea, and nasal congestion, which can be allergic, nonallergic, infectious, occupational, or hormonal in nature. Recently Dykewicz and Hamilos (2010) suggested that in approximately 44–87% of patients with rhinitis, the condition is either allergic or non-allergic in nature. Common allergens causing the problem include proteins and glycoproteins in airborne dust, mite fecal particles, cockroach residues, animal dander, moulds, and pollens. Within minutes of inhalation, these allergens are recognized by IgE antibody bound to mast cells and basophils, causing degranulation and release of preformed mediators, such as histamine and tryptase, and inflammatory mediators, such as leukotrienes and prostaglandin D2. These mediators cause plasma extravasation from blood vessels, with consequent edema, pooling of blood in cavernous sinusoids, and occlusion of nasal passages. These developments are accompanied by active secretion of mucus from glandular and goblet cells, and release of histamine, which manifests as itching, rhinorrhea, and sneezing. In late stages, a prominent nasal congestion develops, as these early phase reactions set off by around 4–8 hours (Rosenwasser, 2007). Rhinitis is often accompanied by non-nasal symptoms, such as allergic conjunctivitis, and it is frequently associated with allergic asthma, pathophysiologically and epidemiologically.
Diagnosis should include evaluation of all specific symptoms and the pattern of occurrence of the symptoms (infrequent/intermittent, seasonal and perennial) along with identification of predisposing factors, previous responses to medication, and coexisting diseases. A handheld otoscope or head lamp with nasal speculum permits viewing of the anterior third of the nasal airway, including the anterior tip of the inferior turbinates. Determination of specific antibody involvement can be made through skin testing, which has high sensitivity, and at the same time is simple, easy, and can be rapidly performed (Wallace et al., 2008). The main treatment for rhinitis is directed towards avoidance of the inciting factors, such as allergens and irritants. A multitude of drugs are available for the management of this disease, including antihistamines, corticosteroids, oral and nasal decongestants, leukotriene receptor antagonists, etc. For the orthodontist, making a referral to an otorhinolaryngologist is an important step in managing the condition. The specialist will determine an individualized treatment approach for each patient, based on their responses, preferences, and cost–effectiveness of the treatment options (Dykewicz and Hamilos, 2010).
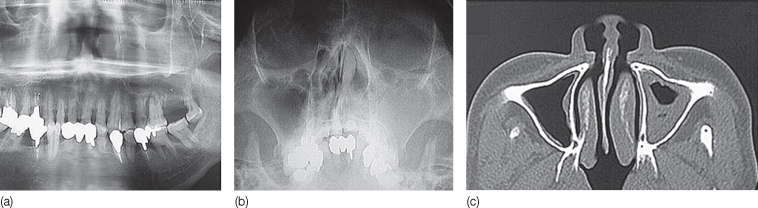
Sinusitis or rhinosinusitis is defined as inflammation of the nose and the paranasal sinuses, and is usually infectious in nature (Meltzer et al., 2004). Typically, sinusitis follows, from a microbiological point of view, a characteristic pattern, starting as a viral infection, followed by superinfection with facultative bacteria after a couple of days and, after 1 week, showing anaerobes as the predominant organisms (Brook, 2009). Acute rhinosinusitis is diagnosed when there is purulent nasal drainage of with up to 4 weeks, accompanied by nasal obstruction, facial pain, pressure, fullness, or both. The pain is usually described as being dull, or felt as pressure in the upper cheeks, between the eyes, or in the forehead. The most common bacteria isolated from sites of acute rhinosinusitis are H. influenzae, Strept. pneumoniae, Moraxella catarrhalis and sometimes Staph. aureus. Disturbances in the sense of smell, which occur in sinusitis, can be either partial (hyposmia) or complete (anosmia), and are usually associated with mucosal thickening or opacification in the anterior ethmoid sinuses, which can be seen a hyperdense areas in an orthopantomograph and computed tomography (CT) images (Dykewicz and Hamilos, 2010) (Figure 14.6). An initial period of watchful waiting without initiation of antibiotics is the best treatment for acute rhinosinusitis.
Figure 14.6 (a–c) Radiological appearances of sinusitis in various radiographs.
(Courtesy of Akitoshi Kawamata, Asahi University School of Dentistry, Gifu, Japan.)
An important aspect of the diagnosis of sinusitis is to differentiate between bacterial and viral causes, as only bacterial infections respond to antibiotic therapy. Clinical signs suggestive of bacterial infection include symptoms that last for more than 7 days. Patients with more severe forms of the disease can be treated with antibiotics, and if this approach is decided on, amoxicillin is the drug of choice. If the patient is allergic to penicillin, a combination of trimethoprim and – sulfamethoxazole (not used in Scandinavian countries) or macrolide antibiotics can be considered. Intranasal decongestants can be prescribed, but for no more than 3 days, to avoid rebound decongestions (Brook, 2010).In contrast to acute rhinosinusitis, chronic rhinosinusitis (CRS) is defined as an inflammatory condition involving the paranasal sinuses and nasal passages over a minimum duration of 8–12 weeks, despite attempts at medical management (Meltzer et al., 2004). Superimposed bacterial infections further complicate the process. The four major symptoms of CRS are: anterior, posterior, or mucopurulent drainage; nasal obstruction or blockage; facial pain, pressure, or fullness; and decreased sense of smell (Meltzer et al., 2004). The bacteria can also form a biofilm over the sinus epithelium. Sequestration of bacteria within biofilms allows the bacteria to resist antibiotic treatment and persist as a low-grade infection within the sinus mucosa. Topical corticosteroid nasal sprays are preferred for all forms of CRS, along with antihistamines for underlying allergic causes, and antibiotics (penicillin V, amoxicillin, or cloxacillin) are prescribed when nasal purulence is present (Dykewicz and Hamilos, 2010). Functional endoscopic sinus surgery (FESS) is the management of choice of chronic refractory rhinosinusitis.
Epiglottitis
Epiglottitis, or inflammation of the epiglottis, is considered to be a medical emergency, as it can lead to airway obstruction and death. It follows oral infection with H. influenzae (Peterson and Thomson, 1999). This microorganism is abundant in the oral mucosal surfaces and in dental plaque. However, certain capsulated strains are considerably more virulent and are thought to be a major pathogen in the respiratory tract, causing otitis, sinusitis, and pharyngitis. The major symptoms are fever, difficulty swallowing, drooling, hoarseness of voice, and stridor. The diagnosis is often confirmed by examination with a laryngoscope, which will show cherry-red and swollen epiglottis and arytenoids (Jenkins and Saunders, 2009). This condition requires immediate medical attention, with urgent endotracheal intubation, to protect the airway. This procedure should be performed by a competent anesthetist, with the help of a respiratory therapist and otorhinolaryngologist (D’Agostino, 2010). In addition, the patient should be given antibiotics, such as ceftriaxone or chloramphenicol, either alone or in combination with penicillin or ampicillin, for streptococcal coverage.
Chronic Infections with Oral Manifestations
Actinomycosis
Actinomycosis is a chronic disease characterized by formation of abscesses, fibrosis, and draining sinuses (Figure 14.7). The causative agent is the nonspore-forming anaerobic or microaerophilic bacterial species of the genus Actinomyces (most commonly Actinomyces israelii), which are normal inhabitants of cervicofacial, thoracic, and abdominal areas (Rivera-Hidalgo and Stanford, 1999). A similar infection, nocardiosis, is caused by the related facultative Nocardia spp. Cervicofacial actinomycosis is the most common form of the disease (Laskaris, 1996). Actinomyces get access to the host tissues when there is an interruption in the mucosal barrier, caused by procedures such as tooth extraction and endodontic infection and treatment. Following this initial superficial invasion, the infection spreads deep into the tissues and a hard, slow growing, relatively tender swelling may form with multiple drainage areas or sinus tracts. The discharge from these tracts typically contains the visible yellowish colonies of the microorganisms and are termed ‘sulfur granules’. If left untreated osteomyelitis with extensive bone destruction will result (Feder, 1990; Brook, 2008). Diagnosis can be confirmed by isolation of Actinomyces species from clinical specimens. Indirect immunofluorescence can also be utilized for this purpose (Gohean et al., 1990). The demonstration of actinomycotic granules, which consist of tangled filaments of organisms, in the exudates or histological sections, confirms the diagnosis. Actinomyces species are susceptible to several antibiotics such as penicillins, tetracyclines, erythromycin, clindamycin, and ciprofloxacin. Antibiotics need to be given at high doses for longer periods (2–6 weeks) for a complete cure. Surgery might be required in some cases to aspirate or surgically drain the abscesses (Brook, 2008). Consultation with an oral medicine specialist and with an otorhinolaryngologist will be of great help in managing these patients.
Gonorrhea
Gonorrhea is the most frequently encountered sexually transmitted disease, caused by Neisseria gonorrhea, a nonmotile spherical oval coccus. The organism is aerobic, Gram-negative and cannot penetrate an intact stratified squamous epithelium, such as that of the skin and oral mucous membrane (Rivera-Hidalgo and Stanford, 1999). However, it can directly invade the urethra, cervix, pharynx, and conjunctiva, because of the presence of columnar and transitional epithelium in these areas. From these sites, infection may spread readily along mucosal surfaces, or systemically, resulting in disseminated disease (Guinta and Fiumara, 1986). Humans are the only known hosts for N. gonorrhea, and the organism has been cultured from saliva of infected individuals. Twenty percent of patients with gonorrhea have oral, pharyngeal, and tonsillar involvement. The tonsils are red and swollen, with grayish exudates. Lesions in the oral mucosa may appear as fiery red and edematous, and occasionally there may be painful ulcerations. Diagnosis is established by culture and identification of N. gonorrhoeae (Laskaris, 1996; Bruce and Rogers, 2004). Sugar fermentation tests aid in species differentiation, and fluorescent antibody techniques have been used for rapid identification of gonococci. Penicillin is the drug of choice for this infection, and in drug-resistant patients ceftriaxone and ciprofloxacin have been used successfully (Chue, 1975).
Mycobacterial Infections
The most common mycobacterial infections are tuberculosis, caused by the tubercle bacilli, Mycobacterium tuberculosis, and leprosy, caused by Mycobacterium leprae. There are also numerous nontuberculous mycobacteria, such as M. kansasii, M. chelonei, and M. avium – intercellular complex (Weg, 1976; Rivera-Hidalgo and Stanford, 1999), which cause most of the medical infections. These infections are noncommunicable from person to person, and do not require long-term systemic medication as in the case of tuberculosis (Rivera-Hidalgo and Stanford, 1999).
Tuberculosis
Tuberculosis is a chronic infectious disease caused by the airborne mycobacterium tubercle bacilli, and spreads almost exclusively via droplets from a person with active disease. Tuberculosis is still among the most life-threatening infectious diseases in humans and one-third of the world’s population comes in contact with the disease at some stage in their life (Kakisi et al., 2010). It commonly affects the lungs, but can occur in any part of the body, and is characterized by formation of granulomas with caseation necrosis caused by cell-mediated response. Primary tuberculosis is seen in patients who have had no previous exposure to the disease and initially is predominantly subclinical. Secondary tuberculosis is seen in previously sensitized patients (Phalen et al., 1996). Oral lesions are rare (0.1–5%, Kakisi et al., 2010), but if they occur, they appear as chronic, painless, irregular ulcers, with a vegetative surface covered by grayish or yellow exudates. The dorsal surface of the tongue is a common site of secondary tuberculous infection, followed by the palate, gingiva, buccal mucosa, and lips (Hale and Tucker, 2008). Diagnosis is reached based on the medical history, identification of acid-fast mycobacteria in clinical specimens, a positive delayed hypersensitivity skin reaction to purified protein derivative, and chest radiograph findings, which includes infiltrates or consolidations and/or cavities in the upper lungs with or without mediastinal or hilar lymphadenopathy. Recent developments in rapid detection and identification of tuberculous b/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses