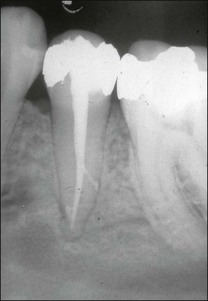
The perio–endo interface
Comparison of apical and marginal periodontitis
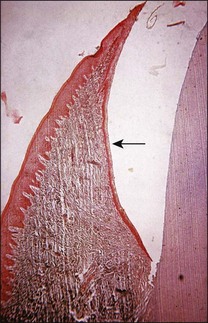
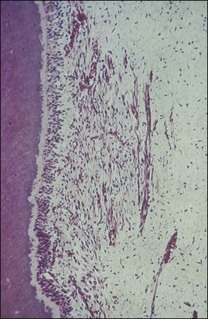
It is a curious and clinically relevant fact that both pulpal and periodontal diseases have their terminal effects in the periodontal tissues (cementum, periodontal ligament, alveolar bone). The difference is that the initial manifestation in periapical disease is at the apex of the root whereas, in periodontal disease, it is at the cervical (marginal) aspect of the tooth (Fig. 12.1). In both cases, the periodontal tissues become chronically inflamed as a result of an anaerobically and Gram-negative dominated microbiota in or on the adjacent root surface.
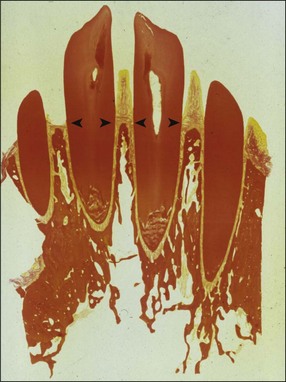
Fig. 12.1 Initial manifestation of periodontal disease at the cervical aspect of the tooth (arrowed)
Preceding disease states in apical or marginal periodontitis
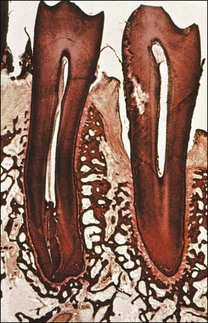
Marginal inflammation of the gingival tissues, called gingivitis, is preceded by the accumulation of plaque around the gingival margin of the tooth. If the plaque is allowed to be sustained, the associated inflammation of the gingival tissues is described to progress through the so-called initial, early, established and advanced lesions. Two types of established lesions are recognized: that which can remain stable for months to years and that which progresses to periodontitis in the susceptible host. The initial defence is mediated at the level of the marginal attachment of the gingival (epithelial and connective) tissues through polymorphonuclear leucocytes (PMNs) and macrophages, as well as inflammation of the underlying connective tissue (Fig. 12.2). In contrast, apical periodontitis is preceded by persistent pulpal inflammation, which follows bacterial colonization of dentine. The initial defence is mediated at the level of the pulp–dentine complex with the odontoblastic layer the key player, together with inflammation of the underlying pulp tissue (see Chapter 1).
Progression, clinical manifestation and measurement of apical and marginal periodontitis
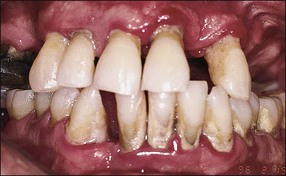
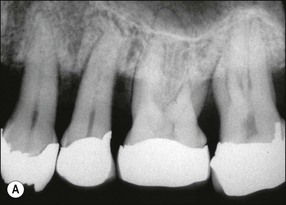
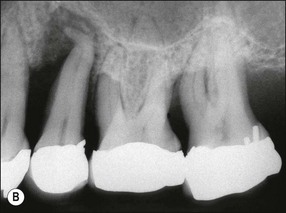
In marginal periodontitis, supragingival plaque sets the stage for subgingival microbial colonization of the root surface. Following the initial signs of gingivitis, which include change in colour of the gingival tissues and bleeding, there is breakdown of the connective tissue attachment and progressive loss of bone when the responsible microbiota are not removed by effective tooth brushing (Figs 12.3–12.5). The degree to which this occurs is determined by modifying or risk factors which affect host susceptibility. Once this process becomes established, the disease progresses apically by periodic exacerbation that may mirror variations in the host immune response. The apical progression involves migration of the junctional epithelium, loss of connective tissue attachment and, ultimately, loss of bone. The pattern of attachment and bone loss can be site specific, varying around the circumference of the same tooth, and/or tooth specific varying around different teeth in the same mouth. The pattern of attachment loss may be relatively constant around all teeth in the mouth resulting in a horizontal pattern of marginal bone loss where the alveolar crest lies more than 2 mm below the cemento–enamel junction but parallel to the occlusal plane (Fig. 12.6). In other cases, the attachment loss may be irregular and localized to specific teeth with angular bone defects (also called vertical defects), in which the pattern of bone destruction progresses vertically along the root creating an oblique orientation of the alveolar crest with the tooth, the crest and root comprising opposite sides of the defect (Fig. 12.7). Although, the exact determinants of the patterns of bone loss remain unclear, this type of attachment loss may be associated with:
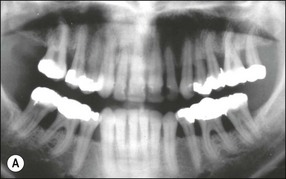
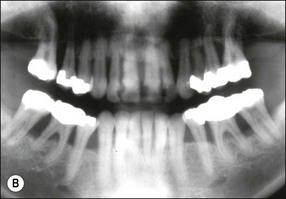
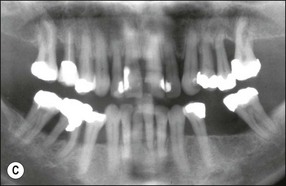
Fig. 12.6 (a) Horizontal bone loss; (b) horizontal bone loss – 4 years later; (c) horizontal bone loss – 12 years later
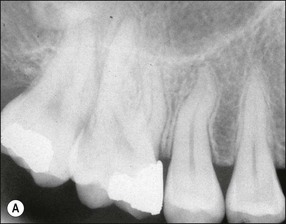
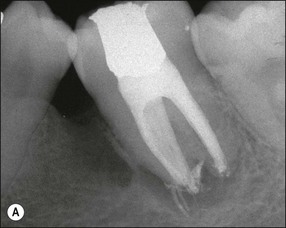
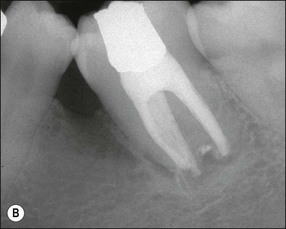
The resulting bony defects take different morphological forms, defined as 1-walled, 2-walled, 3-walled or crater defects. Sites with thin gingival tissue biotype often present with horizontal bone loss and thick biotype with irregular bone loss. Any of these localized forms of attachment loss may eventually encroach on (Fig. 12.8) and envelop the apex of the tooth root (Fig. 12.9) resulting in secondary involvement of the pulp, although this process may take years. Very rarely, periodontal involvement of a lateral canal may cause the demise of the pulp.
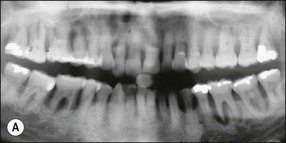
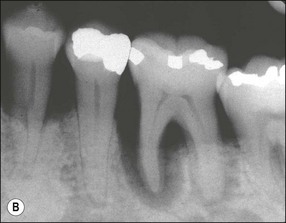
Fig. 12.9 (a) Periodontal bone loss involving the mesial root of 36; (b) periapical radiograph of the same as in (a)
The diagnostic process includes history, clinical examination of the involved tissues and radiography. In the early stages of the disease, there is loss of stippling of the gingival tissues with associated redness and swelling. The gingival tissues are examined for degree of bleeding by running a periodontal probe in the marginal sulcular tissues; inflamed tissues exhibiting immediate bleeding. The extent and severity of periodontal disease is measured by characterizing the attachment loss, taking account of any recession, periodontal probing defects (signifying separation between soft and hard tissue) and radiographic assessment of the pattern of bone loss. Where there is sufficient thickness of bone, the attachment loss may overtake total marginal loss of bone, creating an infra-bony defect (see Fig. 12.3).
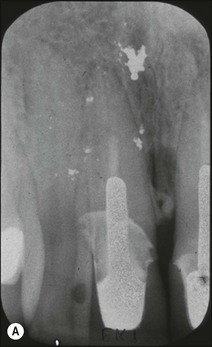
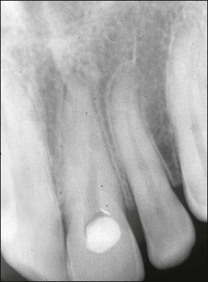
In apical periodontitis, pulp infection commences in the crown of the tooth and progresses apically. If, as on relatively rare occasions, the pulp tissue in a lateral canal becomes necrotic and infected, a lateral periradicular radiolucency (usually enclosed by intact marginal bone) will arise, representing a localized site of inflammation (Fig. 12.10). As the infection in the pulp progresses further apically, the pulp tissue in the apical part of the root succumbs and further lateral and apical foramina may become associated with adjacent bone loss (Fig. 12.11). The size and distribution of apical periodontitis (and thus apical radiolucency) is dictated by:
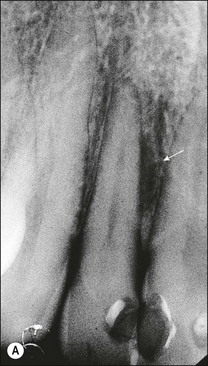
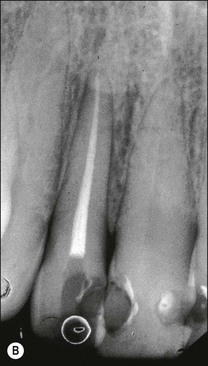
Fig. 12.10 (a) Lateral periodontal bone loss of pulpal origin (arrowed); (b) resolution following root-canal treatment
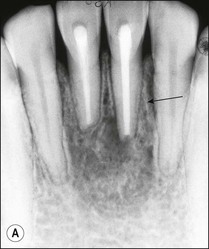
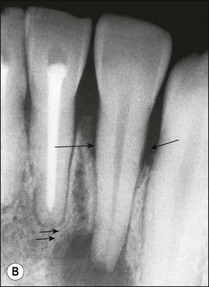
Fig. 12.11 (a) Early periradicular bone loss in 32 (arrowed); (b) further apical and marginal bone loss over a 10-year period in the tooth shown in (a)
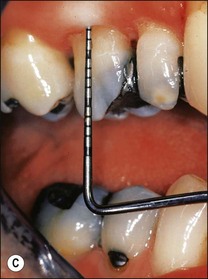
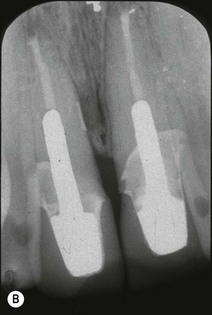
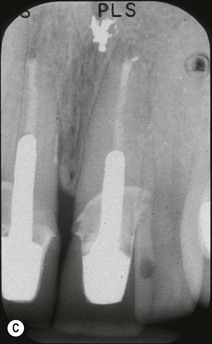
Periapical disease may manifest asymptomatically or symptomatically, depending upon the nature of host and microbial factors involved (see Chapter 3 for details). Periapical lesions can remain asymptomatic and stable in size for years. The presence and extent of periapical disease is mainly measured radiographically (as it is not directly visible or probeable) and also requires standardized radiography. The lesions of chronic apical periodontitis seldom result in retrograde marginal periodontitis with attachment loss. However, when this does occur, the probing depth and bone defect is usually deep and very localized. Long-standing, untreated, marginal loss of attachment caused by root-canal infection may secondarily become established as true marginal periodontitis requiring endodontic and periodontal treatment. It has been suggested through classifications that such periodontal lesions caused by root-canal infection may progressively widen with time. If true, characterization of such loss of attachment requires the recording of the continuous probing profile around the tooth (Fig. 12.12), which defines both the width and the depth of the defect. Following root-canal treatment, the chances of resolution of this type of localized marginal loss of attachment are high, because the infection is “closed” and “contained” within the tooth. The true prevalence of this type of presentation and its susceptibility to treatment is unknown.
Cell profiles in apical and marginal periodontitis
Both disease processes are characterized by transition from innate to adaptive immune responses. The precise distribution of cell proportions varies with time according to the nature of host–microbial interaction. The cell types for apical periodontitis were reported in Chapter 3, and include, PMNs, macrophages, fibroblasts, B and T lymphocytes, mast cells, plasma cells and epithelioid cells. The same cell types are found in the developing lesions of marginal periodontitis, however, the dominant cell type in the advanced periodontal lesion is the plasma cell. Epithelial proliferation is evident in both disease processes, the junctional epithelium in periodontal disease, and the rests of Malassez in apical periodontitis, which may lead to cyst formation in apical but not marginal periodontitis.
Microbiota associated with apical or marginal periodontitis
The details of the microbiota involved in apical periodontitis were discussed in Chapter 3. Both disease processes show the classical progression from an initial Gram-positive, aerobic/facultative colonizing flora, replaced by an increasingly diverse and complex facultatively anaerobic flora, to be succeeded by a progressively strictly anaerobic and Gram-negative dominated microbiota. The diversity of the microbiota in apical periodontitis is more selective and restricted because of the nature of the niche. The dominant strains may be similar in general terms but the species associated with disease progression may be different by virtue of ecological differences. Perio–endo cases show a similarity in the general profile of species recovered from the root canal versus the periodontal pocket but there may be subtle differences at a strain level, even if the same species may appear to be present. The differences may reflect the “open” versus “closed” nature of the niches. Abscesses of apical or marginal origin may have a distinct proportion of spirochaetes according to one source (up to 10% in periapical and up to 60% in lateral periodontal). Research on marginal periodontitis has focused on identifying key species associated with disease progression but equivalent research on apical periodontitis is missing.
Risk factors for progression of apical or marginal periodontitis
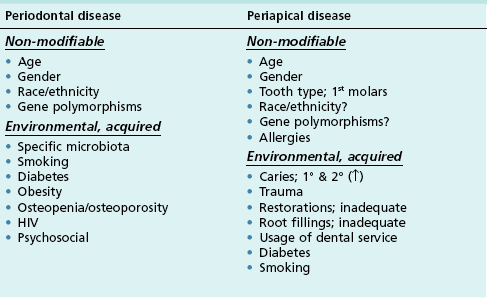
Epidemiological studies on these diseases have allowed various associations to be measured in populations. These yield interesting links between various factors and prevalence or progression of the disease. The types of associations have been divided into non-modifiable and environmental (Table 12.1); although some common factors have been investigated, differences emerge in the pattern of factors that may predispose to each disease’s prevalence or its respective progression. The differences may also reflect the different pathways of exposure to the oral environment.
Association of apical and marginal periodontitis with systemic diseases
In contrast, the number of investigations on the relationship between apical periodontitis and systemic diseases is sparse and without any significant associations. Investigations on the effect of smoking, diabetes and on cardiovascular disease have failed to note significant findings. This may also be explained by the fact that the likely surface area of exposure, between an ulcerated periapical lesion and the resident microbiota in the root canal, is probably limited. It may, however, be argued that the selective and protective sanctuary of the root-canal system may allow privileged entry for uniquely adapted strains, capable of colonizing and invading body tissues; but this remains to be shown.
Pathways of communication between pulp and periodontium
Lateral and accessory canals
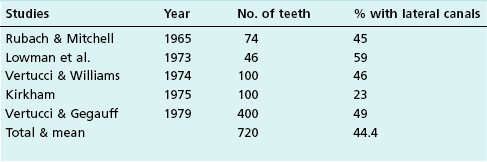
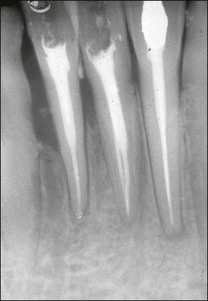
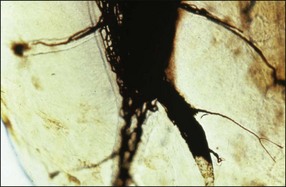
Lateral canals are considered to be rare in the coronal third of the root and arise with a frequency of ≈10% in the middle third (Fig. 12.13) and ≈25–35% in the apical third (Figs 12.14, 12.15) of the root. The mean prevalence of lateral canals, derived from a number of studies as shown in Table 12.2, is ≈45%.
Dentinal tubules
Under normal circumstances, dentinal tubules do not provide a communication between the pulp and periodontium but may do so if, under conditions of gingival recession or loss of attachment, the cementum is worn away by professional or home root debridement (Fig. 12.16). Dentinal tubules provide long and extremely narrow channels of communication (see Chapter 1) but are perfectly capable of conveying PMNs, bacteria and their products, as well as molecular mediators of inflammation (Fig. 12.17) (see Cementum defects).
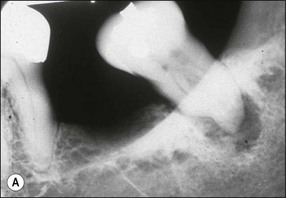
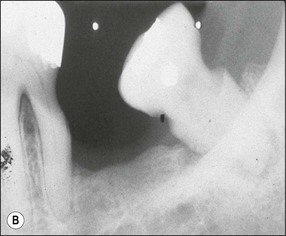
Fig. 12.16 (a,b) Cementum and dentine worn away by professional or home root debridement; note the reduction in the apparent periapical lesion solely through oral hygiene measures – this was not a true periapical lesion (a) but periodontal bone loss superimposed on the periapex (courtesy of Dr Graham Bailey)
Development defects
Various defects, such as invaginations of the coronal or radicular tissues may occur during the development of teeth (see Chapter 1). In the majority of cases, the invaginations do not allow communication between the tissues but, occasionally, developmental grooves, e.g. palato-gingival groove may be deep enough to communicate with the pulp (Fig. 12.18).
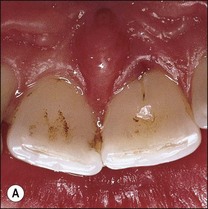
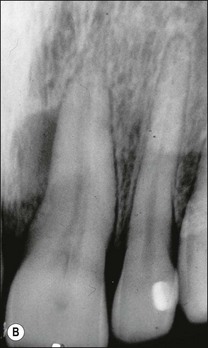
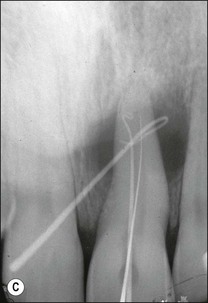
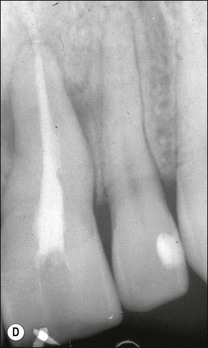
Fig. 12.18 (a) Palato-gingival groove in maxillary central incisor; (b) radiograph of the tooth showing bone loss; (c) diagnostic endodontic instrument in canal and gutta-percha points in sinus tract and periodontal pocket, respectively; (d) radiograph demonstrating some infilling of bony defect but failure due to communication between groove and root canal system
Cementum coverage defects
In 10% of teeth (see Chapter 1), the cementum does not abut or overlap the enamel, leaving dentinal tubules open at the cervical margin of teeth. Equally, such defects may sometimes arise on the root surface through abrasion or as a result of resorption.
Another recognized condition is the cemental tear. These occur mainly in men and may be associated with acute or chronic trauma. They can result in periodontal inflammation, abscess formation and deep probing defects. The majority of involved teeth give positive pulp responses but they may also be found in root-treated teeth with posts. Stress concentration related to the apical extent of the post may result in a crack within the cementum (Fig. 12.19a), as in the shown case, with associated lateral radiolucency that resolved upon surgical cleaning of the crack and filling with EBA cement (Fig. 12.19b,c). The tooth also had apical retrograde root-canal treatment.
Iatrogenic perforations and root fracture
Iatrogenic perforations may occur in lateral walls of the roots or through the pulpal floor in a multirooted tooth as a result of faulty root-canal treatment (furcal strip perforation, apical transportation, lateral perforation) or restorative procedures (post or pin perforations). In addition, root fractures, both vertical and horizontal, create an artificial communication between the pulp space and the periodontal ligament, which may be associated with healthy or root-treated teeth.
Effect of pulp disease and its treatment on the periodontium
Pulpo-periapical inflammation and bone loss
It is well known that pulp inflammation can be transmitted through lateral communications to the periodontium, both in the furcation and apically; when severe, it can manifest in submarginal bone loss, especially in younger patients (Fig. 12.20). Pulpitis is succeeded by pulp necrosis, root-canal infection, and apical periodontitis; the latter may manifest in a number of ways in the periodontium. It may contribute to horizontal marginal bone loss (see Fig. 12.13), vertical intra-bony defects (see Fig. 12.11b), or furcation involvement in multirooted teeth (Fig. 12.21).
Pulpo-periapical inflammation and periodontal wound healing
Some periodontists may request root-canal treatment on teeth with “doubtful” pulp status when regenerative surgery is planned in the site. The rationale is to eliminate possible sources of infection to maximize the potential for successful periodontal outcome. The desire to eliminate potential, unconfirmed root-canal infection must be tempered by the knowledge that extrusion of root-canal treatment materials may have an equally negative impact on periradicular healing (Fig. 12.22) and, therefore, the prognosis of subsequent periodontal surgery. Equally, some periodontists may request root-canal treatment on teeth with “doubtful” pulp status when implant fixture placement is planned in an adjacent site.
Effect of iatrogenic problems
Iatrogenic perforations create additional, and usually larger, channels of communication between the pulp space and periodontium. If previously infected, these may cause insurmountable inflammation that may be treated only by root amputation or tooth extraction. The larger is the communication, the more difficult, the management (Figs 12.23, 12.24).
Effect of periodontal disease and its treatment on the pulp
Effect of periodontal disease on the pulp
Biofilm contamination of the root surface and infection of the periodontium appears to have only a limited effect on the pulp. This is probably because the pulp–dentine complex response tends to close off channels of communication by sclerosis of dentinal tubules (Fig. 12.25) and by laying down secondary dentine. A chronic pulp response may be reflected in a greater prevalence of dystrophic pulp calcification and canal narrowing in periodontally involved teeth, which can lead to technical problems during root-canal treatment. Occasionally, the bacterial stimulus from exposed lateral canals may be sufficient to cause localized pulp necrosis and infection, adding to the periodontal problem. The pulp may eventually succumb either because the periodontal disease reaches the root apex or the infection in the pulp spreads (Fig. 12.26). In either case, the long-term prognosis of the tooth is likely to be poor at this point and it should be extracted or, if a single root of a multirooted tooth is involved, root amputation may be considered.
Effect of periodontal treatment on the pulp
The treatment of marginal periodontitis involves patient education and oral hygiene instruction followed by a course of non-surgical debridement. Previously, the term root planing was used to describe this non-surgical phase of treatment as the aim was to remove the infected cementum layer on the root surface to encourage healing and attachment. The contemporary aim of root surface debridement is to disturb the bacterial biofilm and remove any plaque-retaining factors, such as calculus. However, this in itself inevitably may result in some removal of the cementum layer, possibly exposing dentinal tubules. Microbial colonization of exposed tubules can lead to localized pulp inflammation (Fig. 12.27) that would usually resolve if the pulp is not already compromised by restorative procedures. If compromised, then the microbial insult may be sufficient to tip the balance towards pulp necrosis and infection. Exposure of dentinal tubules leads to dentine sensitivity and with plaque accumulation to hypersensitivity, which is a hyperalgesic state of the former.
Definition and classification of perio–endo lesions
Definition of perio–endo lesions
• an isolated, usually narrow, deep probing depth of pulpal or periodontal origin, in an otherwise periodontal disease-free mouth
• lesion with submarginal or intra-bony periradicular bone loss of pulpal and/or periodontal origin that communicates with the oral cavity via a periodontal probing defect
• a localized deep periodontal probing defect of pulpal or periodontal origin, in a mouth with other sites of periodontal disease, which may or may not be extensive.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses