13 Neurotoxins in Cosmetic Facial Surgery
Botulinum Toxin Type A: History and Physiology
As the Clostridium bacterium was studied and the toxin isolated, it was largely controlled by the U.S. government and considered for chemical warfare but reportedly could not be aerosolized. The government allowed academic investigation in the late 1940s. Dr. Allen Scott (Figure 13-1, A) had the biggest part in the medical progression of the drug, and around the early 1980s the drug, called Oculinum at the time, was used in studies as a treatment for strabismus and blepharospasm. Dr. Jean Carruthers is an oculoplastic surgeon in Vancouver, BC, and was part of the research using botulinum toxin A for the treatment of blepharospasm. She noticed that the drug was successful in treating the muscular disorder, but some patients were returning without lateral canthal wrinkles on the treated side and were inquiring about using it to “make the wrinkles go away” on the nontreated side. Dr. Jean shared this experience with her husband, Dr. Alastair Carruthers, an accomplished dermatologist who was intrigued with this finding (see Figure 13-1, B). One day, Dr. Alastair electively injected one of his employees in the glabellar region with the botulinum toxin, and the first cosmetic treatment of Botox was born.1
Botulism is not an infectious disease but rather a type of food poisoning caused by eating food in which C. botulinum has grown and produced toxin. Botulinum toxin has been called “the most poisonous of all poisons”2 and molecule for molecule is the most lethal substance known to man. The U.S. supply of botulinum toxin A (BTA) that lasted for 2 decades was synthesized in a 150-mg batch by Schantz in 1979.3 This batch of crystalline BTA was dubbed batch 11-79. BTA is one of the eight known toxic serotypes produced by Clostridium botulinum that have been purified (A, B, C1, C2, D, E, F, and G). Seven of the eight serologically distinct toxins can produce neuromuscular blockade, although type A is the most potent. This purified serotype of BTA is available in the United States as Botox (Allergan Inc., Irvine, CA) and Dysport (Medicis Inc., Scottsdale, AZ) and is supplied in a hemagglutinin complex in the form of a freeze-dried powder ready for dilution with preserved normal saline.
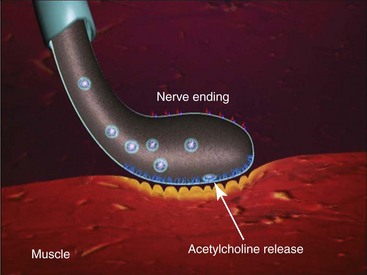
Due to the extreme potency of this exotoxin, the 100-mg Botox vial content is barely visible as a light coat of precipitate adherent to the floor of the glass vial. The FDA requires specifications of 100 plus or minus 30 units per vial. Allergan indicates specifications closer to 100 plus or minus 10 units per vial. These variations may affect the clinical action, onset, and duration of the treated areas and must be kept in mind: 20 units of Botox are equipotent to 60 units of Dysport, Dysport and these differences must be kept in mind when interpreting the literature.4 Having said this, each different type of Botulinum toxin A is different and technically no official conversion rate exists. In my clinical experience and opinion I equate 20 units of Botox to 60 units of Dysport and hence a 3:1 ratio. It is commonly said that there is a 2.5 : 1 ratio of units between Dysport and Botox, but in my personal experience a more accurate conversion is 1 Botox unit is equal to 3 Dysport units. The LD50 for BTA in mice is 1 unit and is expressed as the amount of toxin injected intraperitoneally that kills 50% of a group of Swiss-Webster female mice weighing 18 to 20 grams.5 The LD50 for humans has been calculated at 2500 to 3000 units for a 70-kg person for a lethal dose of 40 U/kg.6 Since the usual therapeutic dose for the treatment of hyperfunctional muscle lines is 25 to 50 Botox units, a 100× margin of safety exists. The packaged exotoxin is stored in a conventional refrigerator and once reconstituted is stored in the same manner. BTA is ordinarily incapable of crossing the blood-brain barrier and generally exhibits no systemic effects.7 BTA is a neurotoxin and exhibits it effects on the neuromuscular junction by inhibiting the release of acetylcholine (ACh) , which causes weakness or flaccid paralysis (Figure 13-2).
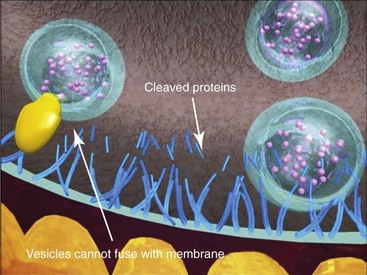
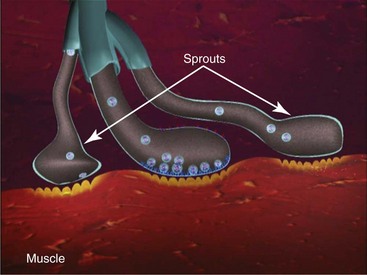
The storage or synthesis of ACh is not affected by BTA; its action affects the vesicle-bound ACh.8 BTA paralyzes all striated muscle exposed to this neurotoxin. The normal presynaptic membrane contains membranous vesicles poised to release their neurotransmitter cargo of ACh at the motor end plate (see Figure 13-2). This process requires a calcium influx and specialized proteins such as SNAP 25 and syntaxin, which make up a “snare complex” that drives the vesicles to the nerve membrane. Normally the vesicles allow passage of ACh through the nerve membrane into the synaptic cleft and through the synaptic junction to bind with specific receptors on the striated muscle, hence causing muscle contraction. When a neurotoxin is injected, the toxin contacts the motor end plate, binds to the neuron (Figure 13-3, A), becomes internalized in vesicles, and moves throughout the neuron (see Figure 13-3, B). The light chain of the toxin (which contains the zinc-dependent protease) produces proteolytic cleavage of the snare complex proteins and prevents the release of ACh at the vesicle membrane, resulting in neuromuscular blockade (Figure 13-4). The binding of the molecule to the motor end plate is permanent; it takes 24 to 48 hours for the therapeutic condition of weakness or paralysis to ensue due to this chemical denervation. The reason for the delay is the time required for the storage vesicles of ACh within the presynaptic motor end plate to be depleted. Although the binding of the ACh is permanent, the paralytic effect only persists for 2 to 6 months. The reason for this temporary action is the formation of new axonal sprouts, thus reestablishing the neurotransmitter pathway (Figure 13-5). This process of neoneurogenesis allows complete recovery of the transmission pathway and resultant muscle function.9
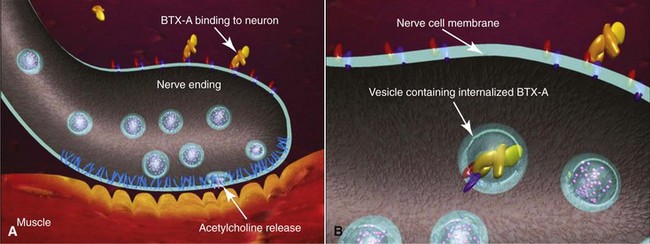
FIGURE 13-3 The toxin binds to the neuronal membrane (A) and becomes internalized in vesicles (B).
(Copyright Allergan Inc., Irvine, CA.)
Resistance to BTA is rare and has been reported with repeated large doses of the exotoxin.10 Resistance from prolonged usage with strabismus treatments appears to increase with the use of more than 300 units within a 30-day period.11 Botulinum toxin serotypes B and F are similarly potent to type A and are being studied for use on patients who have developed immunity to toxin type A.12 Contraindications for BTA include known hypersensitivity to any component of the preparation (including human albumin), systemic neuromuscular diseases, or the use of aminoglycoside or spectinomycin antibiotics which are known to effect neuromuscular transmission and potentiate the effects of BTA.6 Scott13 reported a study of nine pregnant females, one of whom had a premature delivery thought to be unrelated to the treatment. Neurotoxins are never used on pregnant or lactating patients.
Dysport, Dysport, has been used outside the United States for 15 years. It is approved in 73 countries for various therapeutic indications and in 23 countries for the treatment of glabellar or hyperkinetic facial lines.14 Numerous studies have been published in the literature regarding this preparation. One early open-label U.S. study researched a cohort of 1200 patients receiving as many as 5 treatments of Dysport over a 13-month period. The results were strikingly similar to Botox in that the average onset time for effect was 3 days, and the mean duration was 90 days.14 The adverse events associated with this study of over 4000 treatments were not grossly different from Botox studies.14 One difference that must be kept in mind is that the units of delivery of Botox Cosmetic and Dysport are not equipotent. Practitioners must differentiate between the dosages of these different drugs when treating patients.
Clinical Usage of Botulinum Toxin A
I have written numerous articles on the clinical usage of Botox for cosmetic facial treatment, and although my treatment has changed in terms of dilution, my basic injection patterns have remained consistent.15–21 Even though Botox injections are the most popular cosmetic treatment in the world, many patients still do not understand the differences between Botox (or Dysport) and injectable fillers. Realizing this, the surgeon and staff must educate patients on what the drug and treatment will do and won’t do. Like all cosmetic procedures, the consent process is paramount for successful treatment and patient relations. It must be made clear in writing that neurotoxin treatments vary in effect and duration from patient to patient, and that some patients will not respond to treatment. The novice injector will soon find out that some patients will complain that their “Botox did not work” because they can still move their forehead, or the drug did not last long enough, or they had unwanted paralysis. The patient may expect free touchup, retreatment, or a refund because their expectations were not met. For all these reasons, the patient must be made aware of the common effects, unwanted effects, expected duration or lack thereof, complications, patient and surgeon responsibilities, and any financial data that may be relevant. Although many doctors charge by the area, I prefer to charge by the unit. This makes treatment and retreatment simple and understandable for the patient. They are charged for what they receive, and touchups, when required, are simple math. In addition, some patients require more drug than others because they have larger muscles or higher hairlines.
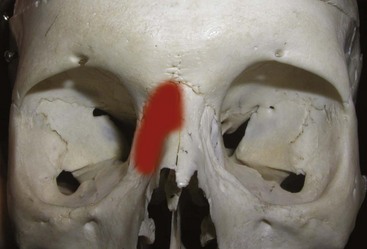
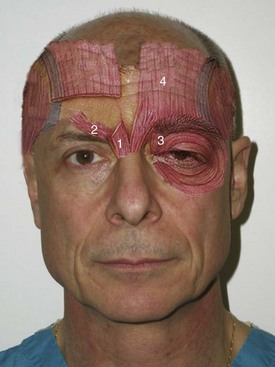
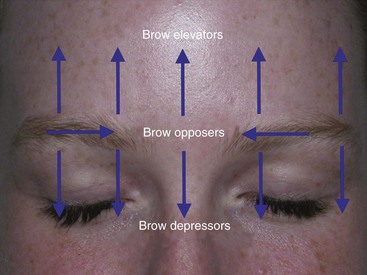
The main depressors of the brow are the paired procerus, corrugator supercilii, and medial orbicularis muscles (Figure 13-6). The sole elevator of the brow is the paired frontalis muscle, and the lateral orbicularis muscles are the main contributors to “crow’s feet” wrinkles. This musculature works in conjunction to perform the very complex orchestra of facial expression (Figure 13-7).
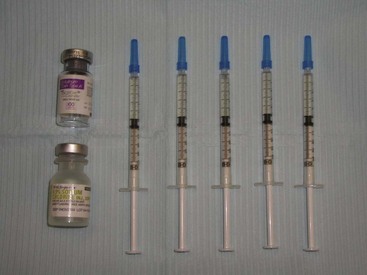
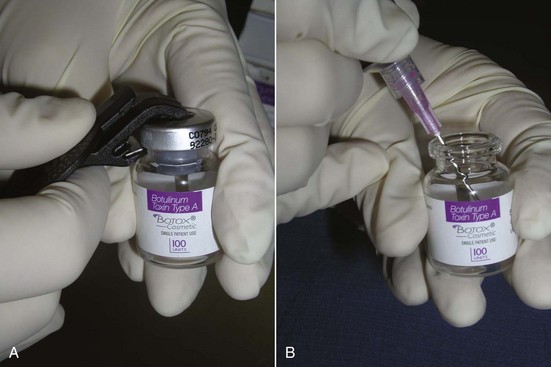
Botox is shipped as a small, thin precipitate on the surface of the vial (Figure 13-8) and is commonly reconstituted with 1 to 5 mL of preserved saline. This is operator dependent and based on the surgeon’s preference. I personally use a 2.5-mL dilution (Figure 13-9). This makes the math easy. There are 100 units of Botox in a vial. I use an 18-gauge needle to inject 2.5 mL of preserved saline into the vial and mix the solution by gently shaking the bottle. The top of the vial is then removed with a bottle opener, and the solution is drawn up in 1-mL Luer-Lok syringes (Figure 13-10). This is done with an 18-gauge needle and never with the fragile needles that will be used for injection. Five syringes are filled to 0.5 mL, which equals 20 units or 4 units per 0.1 mL. This means that each syringe contains 20 units of toxin, and the five syringes total 100 units. Since 20 units are used to treat the average area, this dilution and associated math is simple for staff, doctor, and patients. It is also important to purge the syringes so that the initial injection does not include air instead of neurotoxin.
I prefer 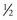 -inch 32-gauge needles (0.26 × 13 mm) and feel their expense is a well-justified price for a less painful injection (Figure 13-11) (Airtite Inc., 1-800-231-7762). It is notable that the more expensive syringes of clear, smooth plastic have much smoother plunger activity thanks to the lubricated stopper (1 mL Luer-Lok Tip Reference #309628, BD Inc., Franklin Lakes, NJ). These are much smoother than the cheaper opaque polyethylene syringes with smaller plunger tips, which are more “jerky” and less precise in terms of injection control. This is especially critical when very small units are administered in precise areas such as the lower eyelid.
-inch 32-gauge needles (0.26 × 13 mm) and feel their expense is a well-justified price for a less painful injection (Figure 13-11) (Airtite Inc., 1-800-231-7762). It is notable that the more expensive syringes of clear, smooth plastic have much smoother plunger activity thanks to the lubricated stopper (1 mL Luer-Lok Tip Reference #309628, BD Inc., Franklin Lakes, NJ). These are much smoother than the cheaper opaque polyethylene syringes with smaller plunger tips, which are more “jerky” and less precise in terms of injection control. This is especially critical when very small units are administered in precise areas such as the lower eyelid.
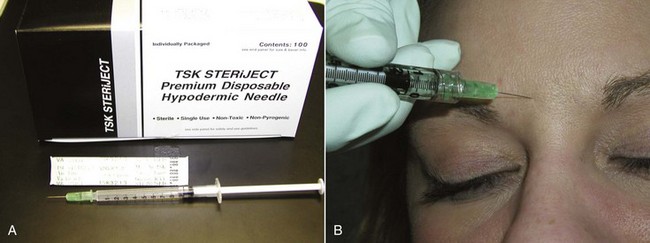
FIGURE 13-11 A Luer-Lok 1-mL syringe with a 32-gauge needle has proven advantageous for accurate and painless injection.
Allergan provides Botox in single-use vials of either 50 or 100 units and originally recommended that the product be discarded at 4 hours; however, the new package insert allows 24 hours after reconstitution. Studies in the literature have shown that 40-day-old reconstituted Botox produces clinical effects up to 40 days.22 Many practitioners will keep reconstituted toxin in the refrigerator for several days or weeks without apparent loss of potency.
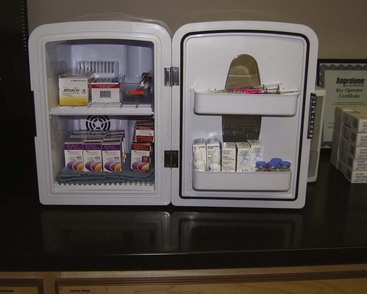
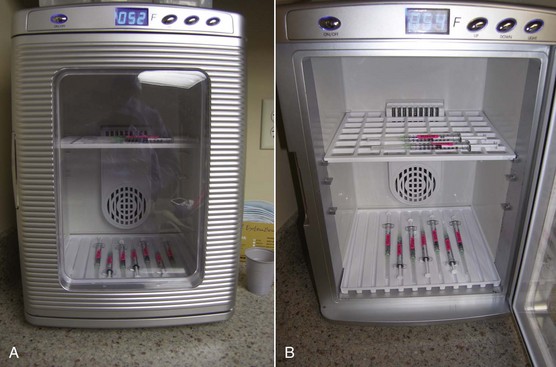
We label every Botox syringe with an appropriate orange label so these syringes are not mistaken for local anesthetic (which has occurred). In addition, a very convenient innovation has been the purchase of small, compact countertop refrigerators. These units are about $100 and not only act as refrigerators but also can be used as heating units for IV fluids and the like. Using these small appliances has made our fast-paced Botox practice much more efficient. The units are aesthetic and occupy a small footprint (Figure 13-12). We use them in our sterilization suite to store our unopened neurotoxin (Figure 13-13) and also keep them in each room where we inject (Figure 13-14). This has saved hours of running around to fetch syringes for use in one of the injection suites and has been a much-appreciated convenience.
Treatment of Glabellar Rhytids
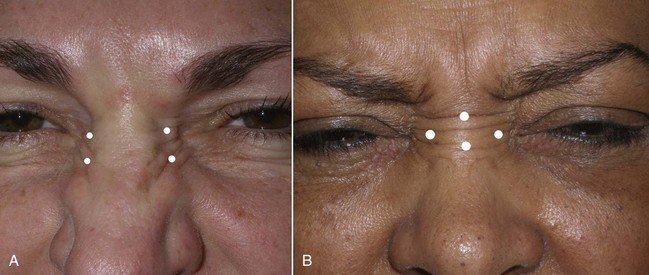
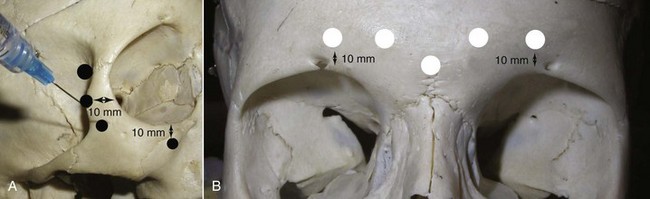
The procerus, corrugators, and medial orbicularis collectively produce the common “frown lines” in the central brow region. The two creases seen in this area are frequently referred to as the 11. The common injection pattern of this region involves five injections placed in a manner to decrease contraction of the depressors. The most important pearl when injecting in the periorbital region is to keep all injections 1 cm from the bony orbital rim. Some injectors choose to inject closer to the orbit, but I can testify that in many thousands of injections adhering to the “10 mm away from the orbit” rule, I have never experienced a true eyelid ptosis (Figure 13-15). I have, however, seen it happen to other injectors when neurotoxin is injected too close to the orbital rim. For the novice injector, it is a good idea to mark out the points of injection with a surgical marker and a ruler or caliper prior to injecting.
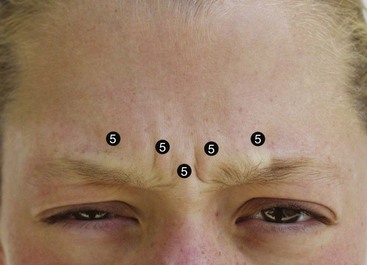
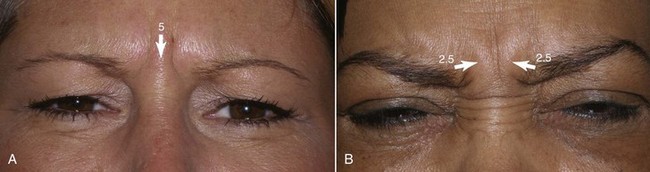
The main injection sites for glabellar rhytids are a central injection into the procerus, an injection in the region of each corrugator supercilii, and a single injection at the action of the medial orbicularis oculi muscle bilaterally (Figure 13-16). I prefer 20 to 25 units to treat this region; when using 25 units, each treatment site receives 5 units. While many patients manifest the procerus action as a single area, it is not unusual to see some patients scowl and observe two separate procerus ridges. For the single ridge seen when scowling, 5 units can be placed centrally; in the case of two separate ridges, 2.5 units can be placed in each ridge (Figure 13-17).
Another relatively common phenomenon is when a patient is asked to frown or scowl, and they recruit both the depressors and the central frontalis (Figure 13-18). This is most frequent in the patient who “does not know how to frown.” Hard to believe that when asked to scowl, some patients do not understand the muscles used to convey that emotion and when asked, exhibit this biphasic expression. If they are handed a mirror and asked to repeat the frown or scowl, most often they will use the pure depressors. If they continue to exhibit this multiple-area depressor/frontalis action, it must be explained to them that they will need both regions treated.
Treating “Bunny” Lines
So-called “bunny lines” are formed by flexing the upper fibers of the nasalis muscle on the lateral nose (Figure 13-19). These lines can be strictly from the nasalis muscle (Figure 13-20, A) or from a combination of heavy procerus action in conjunction with the nasalis (see Figure 13-20, B). Regardless of causation, this area is easily treated by injecting 2-unit aliquots in the circled areas in the figure, which are of course different from patient to patient. Care is used to inject well above the nasolabial fold to avoid affecting lip function. Some patients may not exhibit nasalis activity until their glabella is treated and then recruit the nasalis muscles when attempting to frown.
Frontalis Treatment
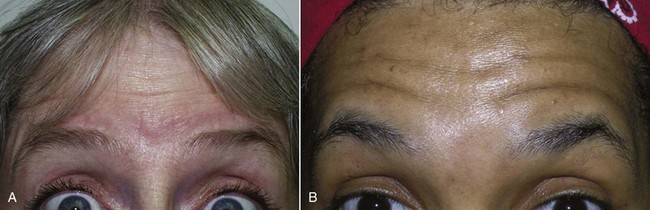
The frontalis is a more variable site than the glabella because hairlines and forehead height are so dramatically different from patient to patient (Figure 13-21). A patient with a very low hairline and short forehead may need very little neurotoxin, whereas a patient with a receding hairline or an elongated forehead may need more than the average patient. In fact, a patient with a very short forehead and low hairline may experience frontalis paralysis when the treatment is limited to the glabella.
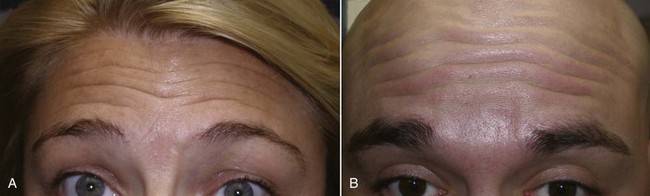
FIGURE 13-21 Patient with a low, short hairline (A) may take much less neurotoxin than one with the inverse (B).
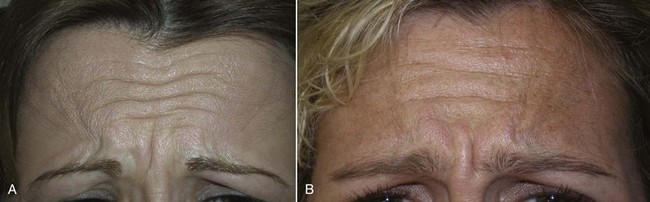
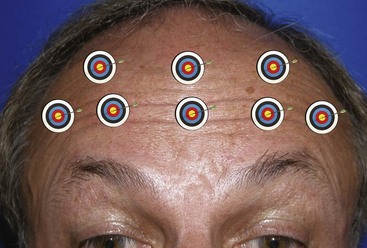
When treating the frontalis, it is important to remember that this is a paired sheet of muscle that can be very thin (Figure 13-22). The actual horizontal forehead rhytids have little to do with the position of the muscle, especially in the midline where many patients have no frontalis. The horizontal rhytids in the skin are perpendicular to the actual muscle, so the treatment is performed by spreading out the toxin over the active regions of the muscle. The injection should be spread out enough to cover all the active muscle area of the broad, thin sheets of the frontalis. It must be kept in mind that when a neurotoxin is injected, the effect fans out like a bull’s eye from the center of the injection for about 10 mm (Figure 13-23). When injecting the frontalis, there is no need to space the injections closer than this, since the effect will spread. When treating the frontalis, a “field” of muscle area is being treated, much in the same way one injects local anesthetic wheals.
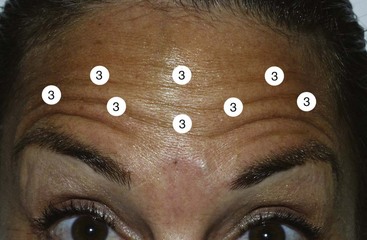
Since the frontalis is a thinner muscle, especially in females, less neurotoxin is required to decrease function. The common dose is 2 to 4 units (Figure 13-24). For patients with very thin skin and muscle, 2 to 3 units per injection may be effective, but for patients with thicker skin and muscle, 4 to 5 units per injection area may be necessary (Figure 13-25).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses





 -inch needle about one-third of its length for the average patient. In reality, neurotoxins have a great affinity for muscle and even if injected intradermally will generally produce the desired effect. A common mistake of novice injectors (especially those used to giving intramuscular injections on the body) is to hold the syringe between their index finger and thumb similar to holding a pencil. They puncture the skin and then have to regrasp the inserted syringe to find the plunger, which is an awkward maneuver. It is better to inject with the thumb on the plunger and the index and second fingers on the hilt of the syringe.
-inch needle about one-third of its length for the average patient. In reality, neurotoxins have a great affinity for muscle and even if injected intradermally will generally produce the desired effect. A common mistake of novice injectors (especially those used to giving intramuscular injections on the body) is to hold the syringe between their index finger and thumb similar to holding a pencil. They puncture the skin and then have to regrasp the inserted syringe to find the plunger, which is an awkward maneuver. It is better to inject with the thumb on the plunger and the index and second fingers on the hilt of the syringe.