Chapter 13
An overview of emergency drugs in the dental practice
INTRODUCTION
The Resuscitation Council (UK) (2012a, 2013a) has provided guidance on what emergency drugs should be available in the dental practice to treat medical emergencies (Box 13.1). All drugs should be stored together, ideally in a purpose-designed container (Greenwood, 2009). The A4 size Emergency Drugs in the Dental Practice guide (Figure 13.1) is a useful aide memoire (drug doses, rotes of administration, etc.) and is designed to be stored in the container containing the emergency drugs. Expiry dates of the drugs should be checked and there should be a planned replacement programme in place (Resuscitation Council (UK), 2012a).
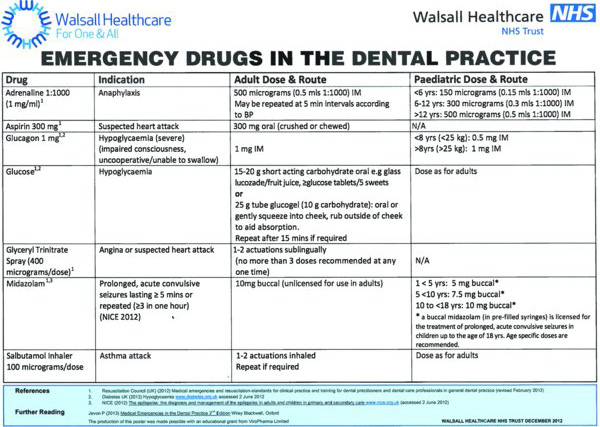
Figure 13.1 ‘Emergency Drugs in the Dental Practice’ guide. Source: Walsall Healthcare NHS Trust. Reproduced with permission.
The aim of this chapter is to understand the use of emergency drugs in the dental practice.
ADRENALINE
Adrenaline is the most important drug in anaphylaxis (McLean-Tooke et al., 2003). Although there are no randomised controlled trials supporting its use, adrenaline is a logical treatment (Brown, 2005) and there is consistent anecdotal evidence endorsing its use to ease breathing difficulty and restore adequate cardiac output (Resuscitation Council (UK), 2012a).
Mode of action
Adrenaline’s alpha-receptor agonist effects reverse peripheral vasodilation and reduce oedema. Its beta-receptor agonist effects dilate the bronchial airways, increase the force of myocardial contraction and suppress histamine and leukotriene release. There are also beta-2 adrenergic receptors on mast cells (Kay and Peachell, 2005) that inhibit activation (Chong et al., 1995), and so early adrenaline attenuates the severity of IgE-mediated allergic reactions (Resuscitation Council (UK), 2012b). Adrenaline seems to be most effective when administered early after the onset of the reaction (Bautista et al., 2002).
Presentation
Pre-filled syringe
Where possible, adrenaline should be in a pre-filled syringe (Resuscitation Council (UK), 2012a). There are currently two products available. UCB’s adrenaline 500 µg product is preferable because this is the recommended does in adults (the graduated syringe also enables use in children).
Ampoules
As repeated dose of adrenaline may be required, most dental practices will also stock adrenaline 1:1000 ampoules that usually come in a box of 10. It is recommended to use a 23 g (blue) needle, or in a larger patient, a 21 g (green) needle, for the intramuscular (IM) injection (Resuscitation Council (UK), 2012b).
Auto-injector devices
Although it would be reasonable to use an auto-injector device if one is immediately available (i.e. patient’s own device), they are primarily for self-use by patients who are at risk of anaphylaxis (Resuscitation Council (UK), 2012b); their routine use by dental practitioners is not recommended because:
- Auto-injectors are relatively expensive with a limited shelf life compared with the cost of an ampoule of adrenaline and syringe and needle;
- Anaphylactic reactions are rare and most auto-injectors purchased for dental practices will not be used;
- Auto-injectors come with standard length of needle which may not be long enough to administer IM adrenaline for some patients;
- Most healthcare staff likely to deal with an anaphylactic reaction in the healthcare setting should have the skills to draw up adrenaline and administer IM injection of adrenaline.
Source: Resuscitation Council (UK) (2013b)
Indication
Anaphylaxis.
Dose and route of administration
- 500 µg (0.5 ml 1:1000) IM.
- The dose is repeated if necessary at 5 minute intervals according to blood pressure, pulse and respiratory function (Resuscitation Council (UK), 2012a).
Paediatric doses
- <6 years: 150 µg (0.15 ml 1:1000) IM.
- 6–12 years: 300 µg (0.3 ml 1:1000) IM.
- >12 years: 500 µg (0.5 ml 1:1000) IM.
Side effects
Side effects include palpitations, dry mouth and tremor. The only reported severe adverse effect following intramuscular administration of adrenaline was a myocardial infarction in a patient with severe ischaemic heart disease (Saff et al., 1993).
ASPIRIN
Aspirin is recommended in a suspected heart attack for its anti-platelet effect. The benefits of administering aspirin are well known. It halves the rate of vascular events (cardiovascular death, non-fatal myocardial infarction and non-fatal stroke) in patients with unstable angina and reduces it by nearly a third in those with acute myocardial infarction (heart attack) (Fox et al., 2004).
Mode of action
Aspirin depletes platelet aggregation and inhibit thrombus formation in the arterial circulation because in faster-flowing blood vessels thrombi are composed mainly of platelets and little fibrin
Presentation
Soluble aspirin 300 mg. It is sometimes stored in a ‘child-proof’ container, in which case it will be necessary to ‘marry-up’ the arrows before it is possible to open the container.
Indication
Suspected heart attack.
Dose and route of administration
- 300 mg crushed or chewed.
Paediatric doses
Not recommended for use in children.
Side effects
Anaphylactic reaction.
Contra-indications
Known allergy to aspirin (NICE, 2012).
GLUCAGON
Glucagon, a polypeptide hormone produced by the alpha cells of the islets of Langerhans in the pancreas, mobilises glycogen (stored glucose) stores in the liver thus increasing the patient’s blood sugar levels. After administration, once the patient is alert and able to swallow, he should be offered a drink containing glucose and if possible some food high in carbohydrate (Resuscitation Council (UK), 2012a).
Mode of action
Glucagon increases the patient’s blood sugar levels by mobilising glycogen in the liver. It should be effective within 10 minutes (Novo Nordisk Ltd, 2013). Glucagon may be ineffective if the patient:
- has been fasting for a long time;
- has low levels of adrenaline;
- is suffering from chronic hypoglycaemia;
- has alcohol-induced hypoglycaemia;
- has a tumour that releases glucagon or insulin.
Source: Novo Nordisk Ltd (2013)
Presentation
Glucagon injection is available as GlucaGen® Hypokit. This comes in an orange tamper-evident container which contains glucagon powder for re-constitution and a syringe with a pre-filled syringe containing water for injection.
GlucaGen can be stored either in a refrigerator (2°C–8°C), or out of a refrigerator below 25°C for up to 18 months within the shelf-life period (Novo Nordisk Ltd, 2013). The advantage of storing GlucaGen with the dental practice’s other emergency drugs is that it keeps all the drugs together in one place. It should be stored in the original package (orange contai/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


