Chapter 12
Synthetic Polymers
12.1 Introduction
Synthetic polymers have been of importance for 60 years or more and find use in almost every sphere of modern life. Prior to the realization of the importance of such products they were often discarded as unwanted byproducts of other chemical processes.
Polymers are high molecular weight, chain-like molecules. A polymer chain does not consist of a random arrangement of atoms, but of distinct repeating groups of atoms, derived from the small molecules or monomers from which the chain is built up. The process by which monomers are converted into polymers is called polymerisation.
Monomers are generally liquids or gases and during the process of polymerisation they become converted to crystalline or amorphous solids. These may vary from being very rigid at one extreme to being soft and rubbery at the other.
12.2 Polymerisation
The conversion of monomer molecules into polymers may proceed by either an addition reaction or a condensation reaction.
Addition polymerisation
An addition reaction simply involves the joining together of two molecules to form a third, larger molecule. For example, ethylene reacts with bromine under the correct conditions to form dibromoethane, as follows.
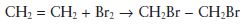
Addition polymerisations involve the addition of a reactive species with a monomer to form a larger reactive species which is capable of further addition with monomer. In simplified terms the reaction may be visualized as follows:
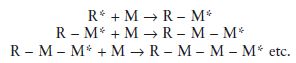
The initial reactive species is represented by R* and the monomer molecules by M. It can be seen how monomer molecules are added during each stage of the polymerisation reaction and eventually a long-chain molecule is produced.
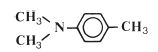
The reactive species which is involved in the addition reaction may be ionic in nature or it may be a free radical. Free radical addition polymerisation is very commonly used for the synthesis of polymers and is the method used in many dental polymers. The free radicals are produced by reactive agents called initiators. These are, generally, molecules which contain one relatively weak bond which is able to undergo decomposition to form two reactive species each carrying an unpaired electron. One very popular initiator, which is used extensively in dental polymers, is benzoyl peroxide. Under certain conditions the peroxide linkage is able to split to form two identical radicals as shown in Fig. 12.1. The decomposition of benzoyl peroxide may be accomplished either by heating or by reaction with a chemical activator. The use of a chemical activator allows polymerisation to occur at low temperatures. Activators commonly used with peroxide initiators are aromatic tertiary amines such as N, N′ dimethyl-p-toluidine (Fig. 12.2).
An alternative activation system involves the use of radiation to cause decomposition of a suitable radiation-sensitive initiator. For example, benzoin methyl ether decomposes to form free radicals when exposed to ultraviolet radiation. Certain ketones, when exposed to radiation in the visible spectrum range and in the presence of a tertiary amine, are capable of forming active radicals which can initiate polymerisation.
Fig. 12.1 Benzoyl peroxide readily splits to form two identical free radicals which can initiate polymerisation.
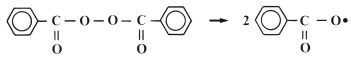
Fig. 12.2 N, N′ dimethyl-p-toluidine – a tertiary amine which is capable of activating peroxide initiators.
The majority of monomers which can be polymerised by a free radical addition mechanism are of the alkene type. That is, they contain a carbon–carbon double bond. These monomers can be represented by the general formula given in Fig. 12.3. Some of the familiar monomers which can be obtained by substituting for X and Y in the figure are also given. Methylmethacrylate and other closely related monomers are of particular importance in dentistry.
The polymerisation processes follow a well-documented pattern which consists of four main stages – activation, initiation, propagation and termination.
Activation: This involves decomposition of the peroxide initiator using either thermal activation (heat), chemical activators or radiation of a suitable wavelength if a radiation-activated initiator is present. For benzoyl peroxide the activation reaction is represented by the equation given in Fig. 12.1. In simplified, general terms it may be expressed as follows:
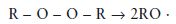
where R represents any organic molecular grouping.
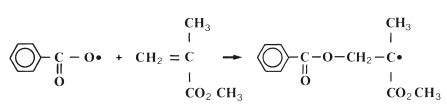
Initiation: The polymerisation reaction is initiated when the radical, formed on activation, reacts with a monomer molecule. This is illustrated for the specific case of the benzoyl peroxide radical and the methacrylate monomer in Fig. 12.4. The reaction may be given in simplified general terms as follows:
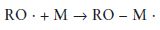
where the symbol M represents one molecule of monomer. It can be seen from the above equation and from Fig. 12.4 that the initiation reaction is an addition reaction producing another active free radical species which is capable of further reaction.
Propagation: Following initiation, the new free radical is capable of reacting with further monomer molecules. Each stage of the reaction produces a new reactive species capable of further reaction, as illustrated in the following equations:
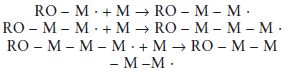
A general equation for the propagation reaction may be written as follows:
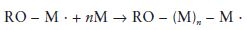
where the value of n defines the number of monomer molecules added and hence the length of the chain and the molecular weight.
Termination: It is possible for the propagation reaction to continue until the supply of monomer molecules is exhausted. In practice however, other reactions, which may result in the termination of a polymer chain, compete with the propagation reaction. These reactions produce dead polymer chains which are not capable of further additions.
One example of termination is the combination of two growing chains to form one dead chain as follows:
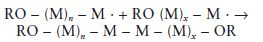
Other examples of termination involve the reactions of growing chains with molecules of initiator, dead polymer, impurity or solvent, if present.
Factors which have an important influence on the properties of the resulting polymer are molecular weight and the degree of chain branching or cross-linking.
Fig. 12.3 General formula for alkene molecules which are capable of polymerizing to form polymers. Examples of some specific monomers are given.
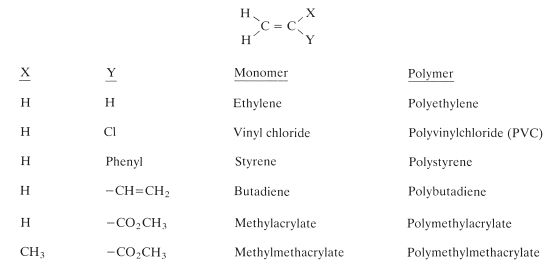
Fig. 12.4 The reaction of a benzoyl peroxide radical with methylmethacrylate to form a new radical species. This is the initiation reaction in free radical polymerisation of methylmethacrylate.
Molecular weight: Within any addition polymerisation system, activation, initiation, propagation and termination reactions occur simultaneously and the resulting polymer is therefore composed of chains of varying lengths. Thus, it is not possible to define a precise molecular weight for polymers and they are normally characterised in terms of an average molecular weight.
Chain branching and cross-linking: Addition polymerisation reactions generally lead to the production of linear polymers. This does not imply that the chains form straight lines but simply that there are no branches off the main polymer chain and that the chains are not linked together.
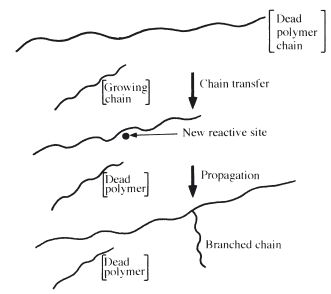
Chain branching may result if a growing chain undergoes chain transfer with a polymer molecule. This involves termination of the growing chain, but a new reactive radical is formed along the side of a polymer molecule. Growth of a fresh chain from this site produces a branched polymer. This is illustrated in Fig. 12.5.
Fig. 12.5 Diagram showing the production of branched polymer chains by chain transfer.
Cross-linking is accomplished by adding cross-linking agents to the polymerizing monomer. In the case of free radical addition polymerisations these agents are invariably difunctional alkenes in which each of the two double bonds present is able to become polymerised into a separate chain, thus effectively linking two chains together. Figure 12.6 shows the general formula for a typical cross-linking agent and the way in which this gives a cross-linked polymer when it becomes involved in a polymerisation reaction. Figure 12.7 gives the structural formula of ethylene glycol dimethacrylate, a cross-linking agent which is commonly used for methacrylate polymers.
Chain branching and cross-linking can have important effects on the properties of polymers.
Although most addition polymerisation processes in dental materials may be characterised as free radical processes, other mechanisms involving the growth of chains through ionic species such as anions and cations are also used. Cationic ring opening reactions involving imine and oxirane groups may be used to produce addition polymers. The ring opening polymerisation of imines is employed in the setting of certain impression materials (Chapter 16) whilst the ring opening polymerisation of oxiranes and closely related siloranes is b/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


