Chapter 11
Transport and postage of diagnostic specimens, impressions and equipment for servicing and repair
LEGAL FRAMEWORK
The transport of infectious items sent by post or courier (e.g. diagnostic clinical specimens, impressions or dental equipment requiring servicing and repair) is governed by the national and international regulations on the Carriage of Dangerous Goods by Road (ADR). These regulations are intended to prevent accidental exposure of people, vehicles or machinery to hazardous materials whilst a package is in transit. In this era of heightened awareness of bioterrorism, correct and safe packaging of infectious items has taken on renewed importance. Compliance with the regulations by the health care community is essential if airlines and courier services are to continue transporting clinical material.
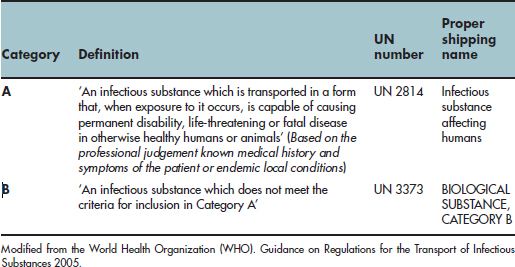
Note that these regulations are based on the United Nations Model Regulations for the Transport of Dangerous Goods, which are revised every 2 years (www.who.int/csr/resources/publications/biosafety). The United Nations (UN) recently altered its method for classifying pathogens for the purposes of international transport. Pathogens will no longer be assigned according to their ACDP Hazard Group; instead they are divided into two new categories, A and B (see Table 11.1). Some substances, however, are not subject to this classification and the exemptions relevant to dentistry are outlined below.
COLLECTING SPECIMENS
The regulations define clinical specimens as any substance, tissue or liquid, removed from the patients for the purpose of analysis. Specimens must be placed in leakproof container immediately after collection, either dry or in transport media, and the lid securely fastened. The patient’s details must be entered on both the outside of the container and the request form. Then the container is placed in a plastic transport bag and the accompanying request form is kept into a separate pouch to avoid contamination.
Table 11.1 UN categories for the transport of infectious substances
Best practice guide: reducing the risk of cross-infection or injury when handling specimens
- Only staff trained to do so should handle specimens
- Specimen container should be placed in the bag separately from the request form and then placed in the designated carrying box. Staples, pins or paper clips should not be used to seal or attach forms to the bag
- Leaking and broken specimen containers should be disposed of as hazardous waste and any spillage cleaned up promptly
- Masks, protective eyewear and gloves should be worn when taking specimens
- Hands should be thoroughly cleaned after handling specimens
- Specimens should not be placed in areas where food is eaten or stored (e.g. kitchen fridge)
TRANSPORT OF SPECIMENS TO THE LABORATORY
Sending non-fixed diagnostic specimens by post
ADR classifications
According to the ADR (2007) classifications, clinical specimens taken from dental patients for diagnostic purposes are assigned to UN 3373 category B infectious substances (diagnostic or clinical specimens). Clinical samples from dental practices transferred to hospital or private laboratories should be packed according to ADR Packing Instructions P650 for road transport or the IATA packing instruction 650 for air transport. These packaging instructions are based on the use of the UN triple-package system (Figure 11.1) which is commercially available from suppliers. Home-made packaging is to be avoided as this is unlikely to be compliant. Packaging for category B infectious substances must be capable of passing a 1.2-m drop test. This means that following a drop from a height of 1.2 m, there is no leakage from the primary receptacle and this should remain protected by the absorbent material within the secondary packaging.
The World Health Organization (WHO) recommends a basic triplepackaging system which is/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


