10
Prevention of Demineralization During Orthodontic Treatment with Fluoride-Containing Materials or Casein Phosphopeptide-Amorphous Calcium Phosphate
Background
It is well recognized that orthodontic appliances can lead to the accumulation of plaque and that plaque is a major etiological factor in the process that leads to demineralization of dental enamel. Orthodontic appliances that are not properly cleaned can therefore increase the incidence of enamel demineralization.
Approaches to reducing the incidence of demineralization during orthodontic treatment have either involved decreasing the amount of plaque (by insisting that the patient maintains good oral hygiene or less commonly by chemical methods) or by tackling the susceptibility of enamel to demineralization. The aim of this chapter is to outline the evidence regarding the effectiveness of the latter approach.
Reducing the Susceptibility of Enamel to Demineralization
It has been known for many years that fluoride reduces the incidence of dental caries (Dean et al. 1950), but it was thought that this was because ingested fluoride ions are incorporated into the hydroxyapatite crystal, making it less soluble when subjected to the acids produced by plaque bacteria. It is now known that the main mechanism by which fluoride works is not systemic, but local, by maintaining the plaque fluoride supersaturated with respect to fluorapatite, hence tipping the balance of the caries process against demineralization and in favor of remineralization (Hellwig & Lennon 2004). This means that topically applied fluoride is a potentially important method of reducing the incidence of demineralization in orthodontic patients, who are well past the age when fluoride can be delivered systemically. In order to be effective, fluoride should be available frequently but does not need to be in a high concentration. Levels as low as 0.01 ppm F promote remineralization (ten Cate 1997).
More recently it has been suggested that the compound casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) may also reduce the incidence of demineralization. The theoretical basis of this arose from the observation that dairy products are anticariogenic. They work in a way similar to fluoride by maintaining the saturation of calcium and phosphate in plaque fluid, thereby discouraging the dissolution of these elements and also promoting remineralization if they are lost (Reynolds 1997).
Methods of Applying Fluoride/CPP-ACP
There are two main methods of exposing the patient to either fluoride or CPP-ACP.
- Topical: In topical form the active ingredient are supplied in forms such as a toothpaste, mouth rinse, gel, varnish, mousse, lozenge, cream or by adding it to milk or chewing gum. Gels and varnishes are usually applied by a professional, particularly if they contain a high concentration of fluoride, whereas the other means of topical application can be self-administered. This has the advantage that the clinician is not relying on the patient to act on the instructions given, but has the disadvantage that the topical form cannot be given frequently, which is preferred in order to promote remineralization.
- Materials Containing the Active Ingredient Fluoride or CPP-ACP can be added to a material used as part of the appliance, either as a bonding or banding material, or an auxiliary such as a glass bead or elastic.
Before examining the evidence that either fluoride or CPP-ACP are effective in reducing the incidence of demineralization during orthodontic treatment, some of the shortcomings of research in this area should be considered.
Limitations of Previous Research
Laboratory Studies
Much of the work examining the effectiveness of fluoride or CPP-ACP in preventing orthodontic demineralization has been carried out in the laboratory. Researchers have attached orthodontic brackets to extracted teeth, subjected the samples to various demineralizing and remineralizing solutions or gels, and measured the amount or depth of mineral loss. The main advantage of undertaking a laboratory study is that this reduces the range and number of variables that might impact the caries process. This will lessen the variability of the data and therefore decrease the number of samples required in order to detect a significant difference. Destructive techniques of determining mineral loss and lesion depth can be used to measure the outcomes accurately, and reproducibly and results are produced quickly and relatively simply.
Unfortunately the strength of laboratory studies, namely that conditions can be controlled very closely, also means that the results are of limited value to the clinician. Laboratory studies are not able to reproduce the many variables that are present in individual patients, such as salivary flow and constituents, dietary (including fluoride) intake, and temperature changes, let alone the difference in susceptibility of each person to caries. Laboratory work is helpful when investigating how materials work and is essential to determine the biocompatibility, but it is of very limited use in assessing clinical effectiveness, which requires well-designed clinical trials.
Clinical Trials—Random or Alternate Allocation?
Some investigators have designed their clinical trials so that experimental and control material is allocated on an alternate basis to patients, arches, or quadrants. Although it could be considered that consecutive patients attending an orthodontist will be fairly randomly distributed with regard to oral hygiene, compliance with instructions, susceptibility to caries, etc., it is important that researchers design their clinical trials so that any possibility of selection or allocation bias is as small as possible. Hence there is a need for detailed inclusion and exclusion criteria for the selection of participants and proper randomization and concealment methods for the allocation to groups.
Short Term or Long Term?
Some clinical studies have only investigated how the material performs in the first few weeks after the orthodontic appliance has been placed (Gorton & Featherstone 2003, Pascotto et al. 2004). This is because they are examining teeth that are due to be extracted as part of the orthodontic treatment plan. Unfortunately, this gives the clinician limited information about how the material will perform over the whole course of orthodontic treatment, which may take many months or years. In the case of materials containing substances that leach out, such as fluoride, it is possible that this will overestimate their effectiveness. We know that there is rapid loss of fluoride in the first few hours and days after it is placed, which is followed by slower and lower levels of loss thereafter. Longer-term studies will also provide information about how the therapeutic agent is lost from the materials, and, in the case of fluoride, if the material also absorbs dietary fluoride into the material (a phenomenon known as “recharge”) and how this affects the demineralization process.
Parallel, Split-Mouth, or Crossover Design?
In addition to the length of the investigation, the results may also be influenced by the design of the study. It is tempting for researchers to undertake a study using a split-mouth or a crossover design, whereby both control and experimental materials are tested in the same participant involved in the trial. In a split-mouth study, the control material is used to bond a bracket to a tooth on one side of the mouth (or arch), and the experimental material is used to bond a bracket to a tooth on the other side of the mouth (or arch). In a crossover study a topically applied control product may be used for a period of time followed by the topically applied experimental product after a suitable “wash-out” period between them to ensure there are no carryover effects. The sides of the mouth or order in which control and experimental products are supplied should be randomly allocated. The advantage of these designs is that the products are tested within the same mouth, theoretically reducing interparticipant variables such as diet, salivary constituents and flow, and individual susceptibility to caries. The problem with the crossover design is that the product is tested for only a limited part of the time that it takes to carry out orthodontic treatment. The disadvantage of the split-mouth study is the possibility that some of the therapeutic element will cross from the experimental quadrant or arch to the opposite, control quadrant or arch, reducing the difference in the incidence of demineralization between the control and experimental materials. The appropriate design for a clinical trial to determine the effectiveness of products in reducing demineralization during orthodontic treatment is therefore that of parallel groups.
Relevant Outcomes
Researchers have used various ways of studying demineralization and more recently remineralization of dental tissues. Early techniques, such as polarized light microscopy and microhardness testing, have been largely superseded and more up-to-date methods should be used, such as computerized microradiography, tomography, and quantitative light-induced fluorescence (QLF). These techniques have been validated to show small changes in mineral loss and lesion depth.
These methods are appropriate for the cariologist investigating the caries process but are less suited to study the effectiveness of products in preventing demineralization during orthodontic treatment. I suggest that the patient and clinician are more interested in the aesthetic problems associated with demineralization. They want to know firstly if there will be fewer unsightly white spots and, secondly, should white spots occur, will they be smaller or less white (demineralized) and therefore less obvious? The researcher does not necessarily require sophisticated (and expensive) research tools to assess these outcomes. Both the number of new white spots (incidence) and the size and whiteness (severity) can be determined from clinical photographs.
Masking of Patients and Clinicians
Ideally, both patient and clinician involved in a clinical trial should be unaware of whether the patient has been allocated to receive the experimental or the control material (double-blind). This technique is used to reduce the potential for participants to influence the results by changing behavior, for example by taking extra care with oral hygiene practices for their favored product. In the case of a topical application, this is relatively straightforward, as two products can be prepared by the manufacturer that are identical in every way, except that one contains the active ingredient (experimental) and one does not (control). It is harder to do with bonding agents that may have different methods of preparing the enamel and curing the material.
Masking of the person treating the patient might not be possible, but it is essential in all studies that the person assessing the demineralized lesions does not know in which group the patient has been allocated. Clinical photographs or QLF images provide a permanent record that allows suitable masking of judges. They also enable the assessment of repeatability within the same judge and reproducibility between judges, which is another essential component of a clinical study. If the variability of the judges in assessing the presence or absence of a demineralized lesion on duplicate images of the same tooth is greater than the difference in their assessment between the experimental and control material, then the validity of the results must be questioned.
Incidence versus Prevalence
The appropriate primary outcome to determine the effectiveness of preventive products is the number of new demineralized lesions appearing during treatment (incidence). Assessment of teeth only at the end of treatment (prevalence) will obviously not exclude those lesions that were present before the appliance was fitted. This requires the researcher to record the appearance of the teeth both just before the appliance is fitted and very soon after it has been removed. Any delay between taking the photographs and fitting the appliance or between removing the appliance and taking the photographs risks the possibility that new demineralized lesions will appear or that any lesions present will remineralize, and this could alter the results of the trial.
In addition, there are many causes of white lesions on enamel other than from demineralization. Although it would be ideal to confirm that a white lesion is caused by demineralization, perhaps by using a fluorescent technique, an experienced clinician can reliably identify a demineralized lesion occurring during orthodontic treatment. Even an inexperienced person, when shown the pretreatment and posttreatment images together, should be able to determine if there has been any change.
Inappropriate Statistical Analyses
Many studies carried out in this area have used the incidence or prevalence of demineralization on individual teeth as the unit for statistical analysis. An assumption made by most statistical tests is that each unit entered into the analysis is independent of the other units. Unfortunately, one tooth in an individual’s mouth is subjected to similar, if not the same, conditions of saliva, diet, and oral hygiene practices as the tooth next to it. Therefore, the assumption that each tooth within the mouth of a participant in a clinical trial is an independent unit is incorrect. The proper unit for the purpose of statistical analysis of the data generated during a clinical trial in this area is the patient, which further supports the need for a parallel groups design. For further details about an appropriate presentation of the data and statistical analysis please see Box 10.1.
Box 10.1 The Calculation of Relative Risk for the Dichotomous Outcome (presence or absence of new demineralized lesions) in Parallel and Split-Mouth Studies
To assess whether the fluoride-containing experimental material or the non-fluoride-containing control material is more likely to have a new demineralized lesion the relative risk can be calculated.
A 2 × 2 contingency table is constructed.
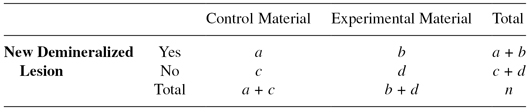
Where:
| a |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


