Chapter 1
The treatment of periodontal disease: the shift from “SRP” to “Periodontal Debridement”
CHAPTER OBJECTIVES
- To provide a historical perspective regarding the development of instrumentation concepts in periodontal therapy.
- To consider the historical focus on endotoxin, and how this led to the preeminence of root planing as a treatment strategy to remove calculus and cementum.
- To consider the role of the plaque biofilm in driving periodontal inflammation, and the importance of the inflammatory host response in periodontal tissue breakdown.
- To explain the current understanding of periodontal pathogenesis and periodontal microbiology, and how this has informed the development of modern periodontal treatment strategies.
- To review the evidence that supports the paradigm shift away from root planing (a damaging form of periodontal therapy) to periodontal debridement therapy (root surface debridement), which achieves the aims of biofilm disruption and removal while at the same time preserving cementum.
Periodontal disease is not new. Archeological investigations have revealed evidence of alveolar bone loss affecting human remains dating from around 700,000 years ago (Dentino et al., 2013). Descriptions of conditions that we would now refer to as periodontitis can be found in a number of ancient textbooks, papyruses, and manuscripts, such as al-Tasrif, the medical encyclopedia written by Albucasis (936–1013) in Moorish Spain. This document was translated into Latin during the twelfth century, and was one of the primary medical texts used in European universities until the seventeenth century (Shklar and Carranza, 2012). In addition to describing the clinical features of periodontitis, some of these early authors also described treatment strategies for the condition. For example, Albucasis focused on the role of calculus in the disease process, and his works included depictions of a variety of instruments for the removal of calculus that bear a striking similarity to many of the periodontal instruments still being used today (Figure 1.1).
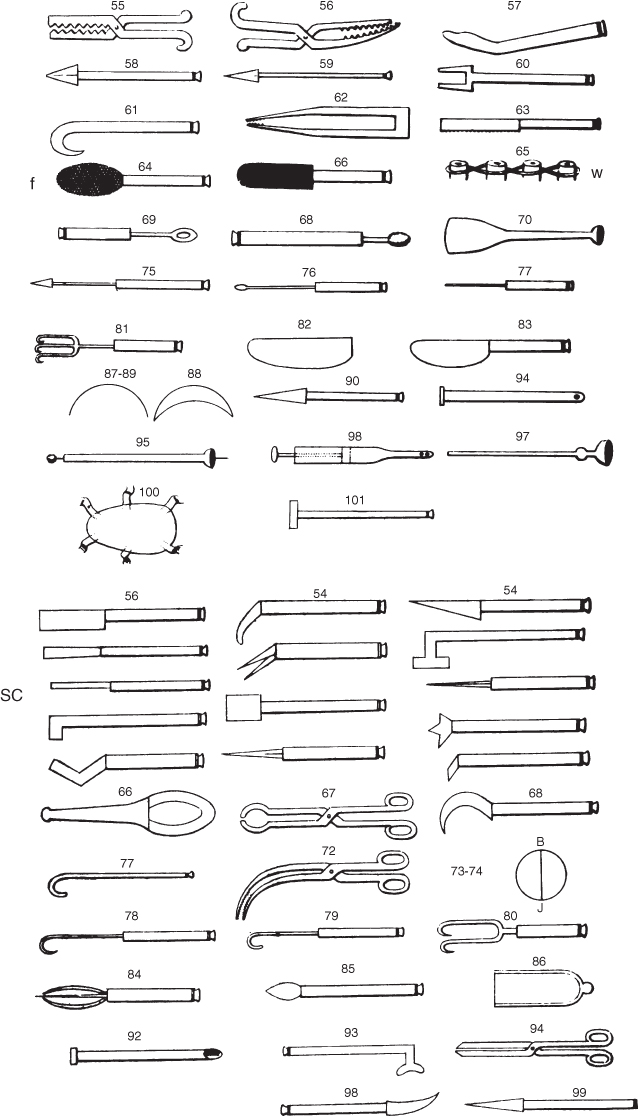
Figure 1.1 Illustration of Albucasis’ periodontal instruments. Note the instruments recognizable as (SC) scalers (left side, halfway down), as well as (f) files (top left, 4th instrument down), blades and scissors, and (w) wiring for mobile teeth (top right, 4th illustration down).
Over the centuries, and more specifically, over recent decades, our understanding of periodontal diseases has evolved exponentially, and as a result, so have the treatment strategies that we employ to manage the condition. Therefore, we no longer treat periodontitis by washing the mouth with wine and water, as advocated by Fauchard (1678–1761), the “father of modern dentistry,” in his 1728 dentistry textbook Le Chirurgien Dentiste. To help us decide which treatment methods are the most appropriate for modern day clinicians to utilize, it is important to briefly review the scientific advances that have been made in periodontology, as these have greatly influenced the treatment protocols that have been used in clinical practice over recent years.
EARLY CONCEPTS OF THE PATHOGENESIS OF PERIODONTAL DISEASE
Calculus the irritant
If we spend a moment to imagine the likely oral health status of many of the people living in the Middle Ages, in the time of Albucasis, for example, we would probably conjure up images of abundant calculus deposits, inflamed gingival tissues, gingival bleeding, and halitosis. It is understandable that these early dentists focused on the role of calculus “accretions” as the cause of the problem, and developed methods for trying to remove the deposits. The etiological role of calculus in the pathogenesis of periodontal disease was unquestioned for many centuries. In the United States of America, Riggs (1810–1885) regarded calculus as the cause of periodontal disease, and treated the condition by the meticulous removal of calculus from pockets, “curettage” of the soft tissues, and oral hygiene instruction (Dentino et al., 2013). For many years, periodontal disease was referred to as “Riggs’ Disease”; such was the influence of this pioneering clinician.
The emergence of microbiology as a discipline, coupled with improvements in microscopy, led to studies of the bacterial composition of dental plaque. The term “pyorrhea alveolaris” was introduced in the late nineteenth century to denote conditions in which gingival pockets developed, which permitted bacteria to “infect and destroy” the periodontal tissues and the alveolar bone. During this era, the importance of local factors in the etiology of periodontal disease was unquestioned, and calculus was viewed as being directly responsible for the tissue damage that was observed in patients with periodontitis. This concept led directly to the emergence of treatment strategies that focused exclusively on calculus removal as the endpoint of periodontal therapy.
The role of plaque
The etiological role of plaque in the development of gingival inflammation was confirmed in experimental studies on gingivitis conducted in the 1960s: upon cessation of oral hygiene practices over periods of 3–4 weeks, plaque accumulation resulted in gingivitis, which was reversed following plaque removal and resumption of normal oral hygiene (Loe and Silness, 1963; Loe et al., 1965). These studies were revolutionary in that they moved the focus of attention away from calculus and more toward plaque as the predominant etiological factor of periodontal diseases.
But how did plaque “cause” periodontal disease? The nonspecific plaque hypothesis made the assumption that periodontal disease (as well as caries) resulted from the production and release of harmful substances from the entire plaque mass. Inherent to this theory were the suppositions (i) that there must be a threshold for these substances (above which periodontal disease will develop, and below which it will not), and (ii) that the amount of plaque present is the main determinant of risk for disease. In other words, this theory suggests that the more plaque a patient has, the more periodontal disease he/she will have. But any clinician knows that this does not hold true; some patients have very poor oral hygiene and lots of plaque, but do not develop advanced periodontitis, and conversely, some patients with good oral hygiene and minimal plaque levels can develop advanced disease.
Further microbiological investigations led to the emergence of the specific plaque hypothesis (Loesche, 1976). This theory held that only certain types of plaque cause disease, because they contain specific bacteria that are particularly pathogenic; for example, they release irritants such as endotoxin, H2S, lactic acid, and bacterial collagenase, which cause injury to the periodontal tissues. It is noteworthy that both the nonspecific and the specific plaque hypotheses considered periodontal tissue breakdown to result from a direct effect of harmful substances released from the plaque bacteria.
Endotoxin
The term “endotoxin” was originally introduced to denote toxic substances within bacterial cells that were released upon death of the bacteria. Today, the term is used synonymously with the term “lipopolysaccharide” (LPS), which is a component of the outer cell wall of gram-negative bacteria. LPS consists of a polysaccharide chain linked covalently to a lipid moiety, and it is essential for maintaining the structural integrity of the bacterial cell wall. LPS induces strong immune and inflammatory responses in higher order species such as humans and other animals, which is why it is so important in the pathogenesis of a number of diseases, including periodontal disease. LPS invokes strong immune–inflammatory responses precisely because it is present in gram-negative bacteria; higher order species have evolved to be able to detect and respond to LPS because it signals the presence of such bacteria.
Research in the 1960s and 1970s identified that endotoxin was present in the outer surfaces of cementum in teeth affected by periodontitis (Daly et al., 1980). It was hypothesized that this endotoxin would limit the effectiveness of periodontal therapy, because even if plaque and calculus were removed from the root surface, the endotoxin still present in the cementum would continue to irritate the tissues and thus compromise healing following treatment. This presumption led to the preeminence of the treatment concept known as “root planing,” often combined as a treatment strategy with scaling, and abbreviated as “SRP” (“scaling and root planing”).
SCALING AND ROOT PLANING (SRP)
SRP became established as a periodontal treatment concept because of the prevailing belief that calculus, endotoxin, and necrotic cementum needed to be removed from the root surface. Necrotic cementum was considered to be that part of the cementum (the outer layer) that was impregnated with endotoxin from the overlying plaque mass. Root planing was therefore employed to vigorously remove this outer layer of cementum by heavy-duty planing of the root surface (think about planing a door to make it fit the door frame better).
What were the objectives of SRP?
We firstly need to decide what is meant by SRP, which actually refers to two separate treatment techniques, “scaling” and “root planing.” Root planing was described as a treatment procedure in the early parts of the twentieth century (Hartzell, 1913; Stillman, 1917) and since then, there have been many different definitions of scaling and root planing in the periodontal literature over the decades. In the 1953 edition of Glickman’s Clinical Periodontology textbook, the use of scalers is described to remove calculus deposits and to “smooth the tooth surface” by the removal of “softened, necrotic cementum” (Glickman, 1953). Root planing is not mentioned at all, but another treatment strategy, “curettage,” is. Curettage was described as the “management of the inner surface of the soft tissue wall” of the pocket, in which the epithelial lining of the pocket was forcibly removed to create a bleeding connective tissue surface against the tooth root, which was believed at that time to result in better healing. This was often achieved by applying pressure to the outside of the pocket (i.e., the free gingiva) with a finger while performing the upstroke with a curet located in the pocket, so that the pocket epithelium was forcibly stripped off. The inner blade of the curet would be removing plaque/calculus/cementum on the upstroke while the outer blade would be stripping off the epithelial lining of the pocket. However, curettage has not been employed for many years, because of the pain it can cause, and also because it was later shown that outcomes following SRP with curettage were the same as those following SRP alone (Echeverria and Caffesse, 1983). Coupled with the confusion that different definitions can create is the further complication that root planing is often accomplished using instruments called curets, and the term “curettage” has sometimes been used interchangeably with the term “root planing.” Curettage is no longer undertaken, and will not be discussed further in this book.
Glickman’s 1953 textbook has evolved over the years, with the most recent version being the 11th edition (2012), now entitled Carranza’s Clinical Periodontology. In that book, scaling is defined as “the process by which biofilm and calculus are removed from both supragingival and subgingival tooth surfaces, but no deliberate attempt is made to remove tooth substance along with the calculus.” Root planing is defined as “the process by which residual embedded calculus and portions of cementum are removed from the roots to produce a smooth, hard, clean surface” (Pattison and Pattison, 2012). In order to clarify matters, our interpretations of these various terms are presented in Box 1.1. Confusion is created, however, by other interpretations and definitions of these terms that are commonly used (Box 1.2 and Box 1.3). In broad terms, and for the purpose of this book, we consider that the objective of scaling is the removal of supra- and subgingival calculus (without damaging the tooth surface), and the objective of root planing is the removal of subgingival calculus together with superficial layers of cementum.
Box 1.1 Periodontal treatment terminology as utilized in this book
- Scaling Instrumentation performed to remove/reduce calculus deposits, both supragingival and subgingival, and without damaging the tooth surface
- Root planing Instrumentation performed to remove subgingival calculus and also cementum (and thereby endotoxin) from the root surface
- Curettage Instrumentation performed to remove the soft tissue lining of the periodontal pocket
- Root surface debridement Instrumentation performed to disrupt and remove the subgingival biofilm, and to remove calculus, but without intentional removal of cementum
Box 1.2 MeSH terms that describe periodontal treatments
MeSH terms
MeSH terms are Medical Subject Headings –they are a series of definitions created by the United States National Library of Medicine (NLM) that are used for indexing journal articles and books.
MeSH terms in relation to periodontal treatment include:
Dental scaling
Removal of dental plaque and dental calculus from the surface of a tooth, from the surface of a tooth apical to the gingival margin accumulated in periodontal pockets, or from the surface coronal to the gingival margin (Year introduced: 1991, updated from 1972).
Root planing
A procedure for smoothing of the roughened root surface or cementum of a tooth after subgingival curettage or scaling, as part of periodontal therapy (Year introduced: 1992).
Subgingival curettage
Removal of degenerated and necrotic epithelium and underlying connective tissue of a periodontal pocket in an effort to convert a chronic ulcerated wound to an acute surgical wound, thereby insuring wound healing and attachment or epithelial adhesion, and shrinkage of the marginal gingiva (Year introduced: 1965).
Periodontal debridement
Removal or disruption of dental deposits and plaque-retentive dental calculus from tooth surfaces and within the periodontal pocket space without deliberate removal of cementum as done in root planing and often in dental scaling. The goal is to conserve dental cementum to help maintain or re-establish a healthy periodontal environment and eliminate periodontitis by using light instrumentation strokes and nonsurgical techniques (e.g., ultrasonic, laser instruments) (Year introduced: 2011).
Authors’ comments
These terms fit reasonably well with modern interpretations. The MeSH definitions of dental scaling and periodontal debridement are closely aligned with our understanding of scaling and root surface debridement as presented in Box 1.1. Importantly, the MeSH definition of periodontal debridement emphasizes that cementum is not deliberately removed, and, importantly, this distinguishes the procedure from root planing. By simply referring to smoothing of roughened surfaces, the MeSH definition for root planing does not adequately convey the damaging nature of the procedure. Clearly, subgingival curettage is an outdated treatment modality.
These terms were taken from the National Center for Biotechnology Information website: NCBI. National Center for Biotechnology Information MeSH Terms [Internet]. 2013 [cited 2013 June 14]. Available from: http://www.ncbi.nlm.nih.gov/mesh/
Box 1.3 ADA Code on Dental Procedures and Nomenclature (CDT) that describes periodontal treatments
CDT Codes
CDT Codes are defined by the American Dental Association and are used by dentists and insurance companies in the United States for coding dental treatments.
CDT Codes in relation to periodontal treatment include:
D4341 and D4342 periodontal scaling and root planing
This procedure involves instrumentation of the crown and root surfaces of the teeth to remove plaque and calculus from these surfaces. It is indicated for patients with periodontal disease and is therapeutic, not prophylactic, in nature. Root planing is the definitive procedure designed for the removal of cementum and dentin that is rough, and/or permeated by calculus or contaminated with toxins or microorganisms. Some soft tissue removal occurs. This procedure may be used as a definitive treatment in some stages of periodontal disease and/or as a part of presurgical procedures in others.
(D4341 is used if there are ≥ 4 teeth per quadrant to treat, and D4342 is used if there are < 4 teeth per quadrant to treat).
D4355 full mouth debridement to enable comprehensive evaluation and diagnosis
The gross removal of plaque and calculus that interfere with the ability of the dentist to perform a comprehensive oral evaluation. This preliminary procedure does not preclude the need for additional procedures.
Authors’ comments
The description of root planing presented here is consistent with the aim of removing cementum, dentine, and calculus that defines this treatment procedure. The description also acknowledges that both hard and soft tissue damage results. The emphasis on removing toxins (i.e., endotoxin) is not consistent with modern understanding of (i) microbial biofilms, (ii) the fact that endotoxin is loosely adherent to cementum, or (iii) periodontal pathogenesis. The description of full mouth debridement here refers to a procedure for removing gross plaque and calculus deposits to enable a complete oral examination. This interpretation is at variance with the modern understanding of the term debridement as shown in Box 1.1. Clearly, the CDT codes will result in most practitioners undertaking periodontal therapy that is destructive to the root surface.
These terms were taken from the CDT Codes published by the ADA: American Dental Association Code on Dental Procedures and Nomenclature 2012 (effective for the calendar year 01 January 2013 through 31 December 2013).
The (historical) justification for root planing
Given the historical belief that calculus was the primary etiology of periodontitis, it is understandable that treatment strategies focused on the complete removal of all calculus. As mandated in 1953, “every speck of it must be removed” (Glickman, 1953). To the modern clinician, calculus is certainly plaque retentive and unsightly, and removing it is an important part of therapy; but, is it really possible to remove every speck of it? A number of studies have addressed this issue. In a study of 690 root surfaces in 11 patients with moderate/advanced periodontitis, the percentage of surfaces with residual calculus following instrumentation with a sonic scaler was 32%, with manual instruments was 27%, and with both instruments used together was 17% (Gellin et al., 1986). The researchers also found that deeper pockets were associated with more residual calculus following instrumentation. In another study, 476 surfaces on 101 extracted teeth were instrumented using both ultrasonic and hand instruments. Following the instrumentation, 19% of surfaces had residual calculus that could be detected clinically, and 57% of surfaces had residual calculus on examination under the microscope (Sherman et al., 1990). In a study of 21 patients requiring multiple extractions, SRP was provided prior to the extractions and the percentage of the subgingival surfaces that were free of calculus was determined with a stereomicroscope (Caffesse et al., 1986). In pockets that were 4–6 mm deep, only 43% of surfaces were free of calculus after closed SRP, and in pockets that were > 6 mm deep, only 32% of surfaces were free of calculus after treatment. The extent of residual calculus was directly correlated with probing depth, and was greatest at the cemento–enamel junction, and in association with grooves and in furcations (Caffesse et al., 1986)
Taken collectively, the outcomes from a number of similar studies that have investigated this issue confirm that nonsurgical instrumentation is effective in significantly reducing the amount of calculus on root surfaces. However, complete calculus removal is not usually achieved, and deeper pockets are more likely to harbor residual calculus following treatment, with anywhere from approximately 3% to 80% of instrumented root surfaces showing some residual calculus after instrumentation (Claffey et al., 2004).
Furthermore, we clinicians are not very effective in determining whether we have fully removed calculus or not (Figure 1.2). For example, in the study described above, there was a high false-negative response rate in that 77% of the surfaces that were determined to have residual calculus when examined under the microscope had been clinically scored previously as being free of calculus (Sherman et al., 1990). This underscores the difficulties of determining the thoroughness of subgingival instrumentation. Calculus detection is technically very challenging due to the complex anatomy of the pocket environment. While supragingival calculus can be identified by direct vision and drying of the tissues, subgingival calculus is much more difficult to identify, unless the deposits are very large and can be detected by a periodontal probe or calculus explorer, or can be seen at the pocket opening. Radiographic detection of calculus is similarly unreliable; a sensitivity for radiographic detection of calculus of only 44% has been reported (i.e., only 44% of surfaces known to have calculus present clinically could be detected on radiographic examination) (Buchanan et al., 1987).
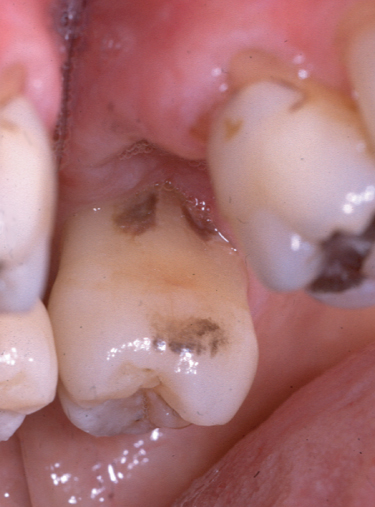
Figure 1.2 Residual calculus that had been missed during previous periodontal instrumentation. This patient had received previous full mouth periodontal instrumentation on several occasions and was in the maintenance phase of therapy. Tooth #14 (FDI 26) was extracted because of endodontic problems, and as the extraction site healed, the tissues at the mesial aspect of #15 (FDI 27) receded to reveal subgingival calculus that had not been removed by the prior periodontal instrumentation (dark brown areas in two locations close to the gingival margin)
Besides calculus removal, the other main rationale that has been given for planing the roots is removal of cementum as the means by which to remove endotoxin (sometimes described as the removal of “contaminated cementum”). Early investigators reported that endotoxin was present in the cementum of teeth affected by periodontitis and that it was biologically active with an inhibitory effect on cellular function (Aleo et al., 1974). Further studies reported that fibroblasts did not attach to periodontally compromised roots until after the cementum was removed, or the endotoxin was removed chemically (Aleo et al., 1975; Assad et al., 1987). These studies implied that for periodontal treatment to be successful, there needs to be meticulous removal of cementum and endotoxin from the root surfaces. Further justification for this approach came from studies that showed that planing the roots resulted in significant reductions in the amount of endotoxin present, typically rendering the root surfaces nearly free of endotoxin (Jones and O’Leary, 1978). It was considered that removing the endotoxin creates a root surface that is more “biologi/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


