Principles of adhesion
Introduction
Since the acid-etch technique of bonding to enamel was introduced into dentistry, the use of adhesive procedures has developed to such an extent that it now constitutes a major part of the dental discipline. Many concepts, which have served the profession well for many decades in providing good dental care, have had to be revised in light of these developments and many new techniques and materials have been introduced.
Two examples of new, adhesive restorative procedures that spring to mind readily are resin-bonded bridges and porcelain veneers. These procedures have been possible because of our improved knowledge and understanding of the surface characteristics of enamel and dentine, and of the requirements that need to be satisfied in order to obtain good bonds to them.
These advances in themselves would not have been sufficient, but they laid a foundation for the development of the new materials and techniques that are used in enamel and dentine bonding today. A combination of factors has provided the dentist with a variety of procedures for restoring the dentition. Although these procedures have been available for only a relatively short time, their impact has already been quite considerable.
There are now many materials that we wish to bond to enamel and dentine, and to each other. Consequently, numerous adhesives have been developed to cope with the diversity of the applications; such adhesives include composite resins, glass–ionomer cements and dentine-bonding agents.
New methods of surface preparation, such as etching and silane coupling, have had to be investigated to find ways of using them in conjunction with materials such as the new glass–ceramics and a wide variety of alloys.
It is the variety of applications that has contributed to the growing complexity of adhesive restorative dentistry. In order to appreciate fully and understand the clinical application of adhesive techniques, it is important for the clinician to have a thorough knowledge of the principles of adhesion, the materials employed, the dental adhesive systems and how these are applied in the clinical situation.
What is adhesion?
Adhesion can be defined as the force that binds two dissimilar materials together when they are brought into intimate contact. This is distinct from cohesion, which is the attraction between similar atoms or molecules within one substance.
Adhesion between solids
At an atomic level, surfaces are rough. This means that, when they are brought into contact, the only places where intimate contact is achieved is at the tips of the asperities (Figure 1.9.1). Very high pressures can be generated at these points, such that, in the absence of any contaminants, an effect called local adhesion or cold welding can result. If an attempt is then made to slide the one surface over the other, a resistance known as friction is experienced.
Friction is caused by the need of the local adhesions to be sheared, or broken. In general, the local adhesions are so strong that the shearing process does not take place at the interface but actually within the solids themselves; this explains the general phenomenon of frictional wear.
While frictional forces due to local adhesion can be quite high, adhesion normal (i.e. perpendicular) to the surface is usually undetectable. This has been attributed to the build-up of elastic stresses in the normal direction, which are released when the load on the material is removed.
Only very soft metals, such as pure gold, can relieve these elastic stresses by flow and prevent rupture of the junction when a normal load is applied. A dental example of this is the use of cohesive gold.
Adhesion between a solid and a liquid
It is a matter of common observation that a drop of water will cling to the underside of a glass slide. This effect demonstrates the adherence of water to glass that arises by virtue of molecular attraction between the two substances. The attraction is due to secondary (van der Waals) bonds. Even a hard shake of the slide will not remove all of the water and merely drying the glass with a cloth will still leave a very thin residual layer of water. The only way of ensuring that all the water has been removed is by heating the glass in an oven.
This illustrates the good adhesion that may be obtained between a solid and a liquid. Such good adhesion is due to the liquid’s ability to make intimate contact with the solid over a large surface area. This is in contrast to the poor adhesion (described above) that usually occurs between two solids, where the contact is at points only.
Thus, one of the fundamental requirements of adhesion is that the two substances to be bonded must be in close contact with each other. The importance of this statement cannot be overemphasized, as a strong bond can be created only in the case of intimate molecular contact. This may seem a simple requirement, but it is not particularly easy to achieve intimate contact at the microscopic level, as noted for solids above.
Given that the distance between the interacting molecules must be less than 0.0007 µm (micrometres; 1 mm = 1000 µm) for adhesion to occur, one appreciates that adhesion is virtually impossible for two solid surfaces. This is a serious obstacle when there is a need for adhesion between two solids, and in order to overcome this, we use a third substance, usually in a fluid or semi-fluid state, to act as an intermediary.
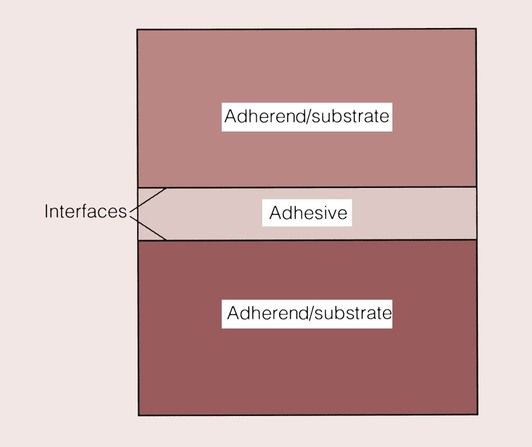
The substance that binds the two materials is defined as the adhesive, and the surfaces of the materials are the adherend or substrate. The point at which the substrate meets the adhesive is described as the interface (Figure 1.9.2).
Naturally, what happens at the interface is crucially important to the success or failure of an adhesive bond. This applies equally to industrial and dental adhesives, so it is useful in the first instance to consider the general requirements of an adhesive and then to look more closely at the bonding mechanisms.
Criteria for adhesion
When reading the instruction leaflet of any adhesive, one sees that one of the first requirements is invariably that the surfaces to be bonded are both clean and dry. This is important for a variety of reasons. A clean, dry surface ensures that the adhesive has the best possible chance of creating a proper bond with the solid material. The presence on the surface of anything that could be considered as a contaminant will prevent the formation of a strong bond, since the contaminant itself is weakly bonded to the solid and will prevent the adhesion of the adhesive to the substrate.
The factors that govern the ability of the adhesive to make intimate contact with the substrate are:
Wettability
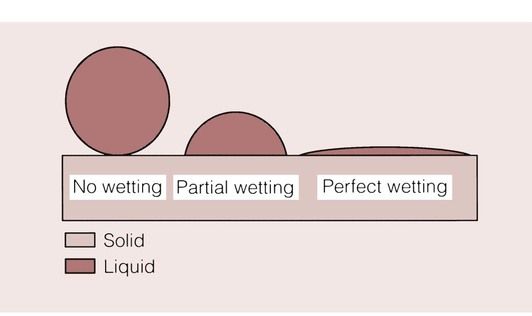
In order for the adhesive to create a bond between two materials, it must make intimate contact with the surfaces of the substrates such that no air voids (which would weaken the bond) are formed. The ability of an adhesive to contact a substrate depends on the wettability of the adhesive on that particular substrate. Good wetting is the ability to cover the substrate completely, so that the maximum benefit is obtained from whichever adhesive mechanism is activated.
The ability or inability of fluids to wet a surface is frequently encountered in everyday life. An example of a surface that is extremely difficult to wet with water is PTFE (polytetrafluoroethylene), as used in non-stick saucepans. When water is placed on a PTFE surface, it forms globules that will not spread in an even layer across the surface. This is an example of poor wettability. This and the other possible responses are depicted in Figure 1.9.3.
The interaction between the substrate and the adhesive is governed by a driving force that tends to spread the adhesive over the substrate, and resistance to spreading that depends on the viscosity of the adhesive, the surface irregularities and the presence of contaminants. The driving force is provided by the surface energies of the adhesive and the substrate (see below).
Surface energy
In the bulk of a solid or a liquid, the molecules are subjected to attractive forces in all directions, such that the molecule is in dynamic equilibrium with its surrounding molecules. At the surface, however, this delicate balance is destroyed, resulting in a net inward attraction directed towards the large number of molecules in the mass of the material. It is this inward force that gives rise to the surface energy of a material. In liquids, the surface energy is known as the surface tension.
One of the effects of surface tension is the tendency for liquids to take up a spherical shape in preference to any other. This arises because a sphere has the minimum surface area (and hence the minimum surface energy) for a given volume of liquid, allowing the total energy stored in the liquid to be a minimum.
Whereas the surface tension of a liquid is a real surface stress, in the case of a solid, work is done in stretching and not in forming the surface. The measurement of the surface energy of a solid is not achieved as readily as it is with liquids. An approach that has now gained wide acceptance is one pioneered by Zisman, who introduced the concept of the critical surface energy.
Contact angle
When a solid and a liquid make contact, the angle between the liquid surface and the solid surface is known as the contact angle, and is dependent on the surface tension of the liquid and the surface energy of the solid (Figure 1.9.4).
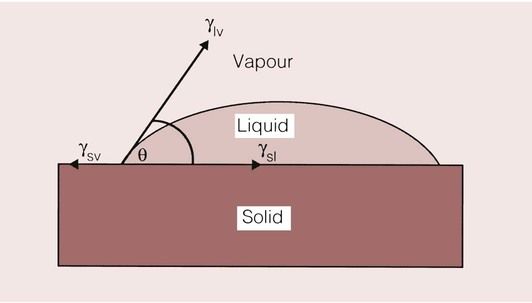
By measuring the contact angle between the solid and the liquid, a useful measure of the wettability of the liquid on a particular substrate can be obtained. For perfect wetting, which is the ideal situation for adhesion to occur, this angle should be 0°. In this case, the surface is completely covered with the adhesive and the maximum bond strength can be achieved. The driving force that gives rise to the tendency, or otherwise, of a fluid to spread on a solid surface depends on the surface tension of the liquid and the surface energy of the solid. At the point where the surface of the liquid meets the surface of the solid, their surface tensions must balance, in order to be in equilibrium:
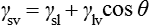
This relationship can be rearranged to give the contact angle, θ, and in this form is known as the Young equation:
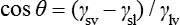
where γsl is the surface energy at the solid–liquid interface, γsv is the surface energy at the solid–vapour interface and γlv is the surface energy at the liquid–vapour interface.
Critical surface energy
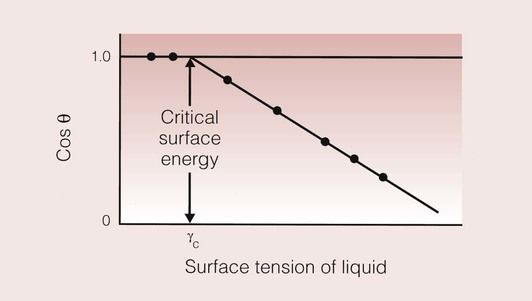
If one measures the contact angle of a number of different liquids on the same substrate and plots the cosine of the contact angle against the known surface tension of the liquids, then a linear relationship results.
This relationship is shown in Figure 1.9.5; it shows the linear curve being extrapolated to the point where it crosses the line at which the cosine of the contact angle is equal to 1. This is the situation under which the contact angle will be 0°, representing the condition of perfect wetting.
The value of the surface tension at which the cosine of the contact equals 1 is defined as the critical surface energy of the solid. This critical surface energy is equal to the surface tension of a liquid that will just spread on the surface of the solid; such a liquid may be real or hypothetical. Any liquid that has a surface tension less than the critical surface energy of the solid will wet the surface of the solid effectively.
Thus, a low surface energy liquid will readily spread over a high surface energy substrate because the surface of the substrate is replaced by a surface with a lower surface energy.
PTFE has a very low surface energy, making it difficult to find liquids with lower surface tensions that could wet it successfully. Another material w/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses