Structure of polymers
Introduction
Plastics and rubbers, as they are generally called in everyday life, have the common property of being polymers. Polymers are long-chain molecules, consisting of many repeating units, as discussed already in Chapter 1.2. Polymers are not a 20th-century invention; they are, in fact, older than human beings themselves, and in one form or another are the basic constituents of every kind of living matter, whether plant or animal.
Examples of naturally occurring polymers are agar, cellulose, DNA, proteins, natural rubber, collagen and silk.
It is only relatively recently that we have begun to understand the structure of polymers and how to make them ourselves. Some examples of synthetic polymers, which are now everyday household names, are PVC (polyvinyl chloride), polyethylene, nylon and polystyrene.
Originally, the synthetic polymers tended to be regarded as substitutes for existing natural polymers, such as rubber and silk. Nowadays, such a wide variety of polymers can be produced that they have entered into every walk of life, satisfying needs that did not previously exist. Pertinent examples are medical applications, such as dialysis and oxygenator membranes, and dental applications such as filling materials.
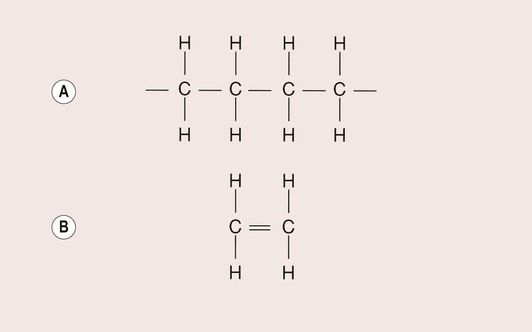
The starting material for the production of a polymer is the monomer. In a material such as polyethylene, the repeating unit is a CH2 group, with many of these units joined together to form a long chain (Figure 1.5.1a). The monomer from which this polymer is derived is ethylene (Figure 1.5.1b).
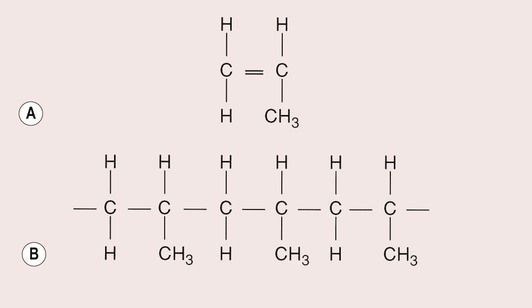
A polymer with a similar structure to polyethylene is polypropylene. It is formed by joining molecules of propylene (Figure 1.5.2a). Propylene differs from ethylene in having a methyl group (CH3) that replaces one of the hydrogen atoms, forming the polymer polypropylene (Figure 1.5.2b).
Polypropylene is slightly more complex than polyethylene, in that the arrangement of the methyl groups can vary so that they:
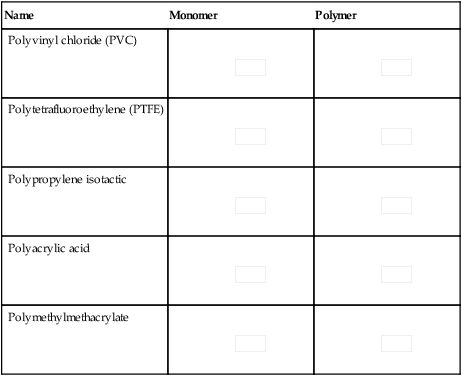
A number of polymers based on vinyl monomers are presented in Table 1.5.1.
Table 1.5.1
Some monomers and their polymers
| Name | Monomer | Polymer |
| Polyvinyl chloride (PVC) |
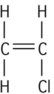 |
 |
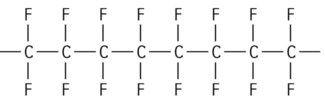
| Polytetrafluoroethylene (PTFE) |
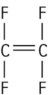 |
 |
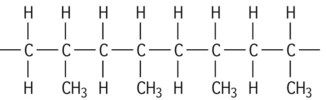
| Polypropylene isotactic |
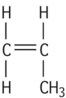 |
 |
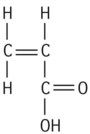
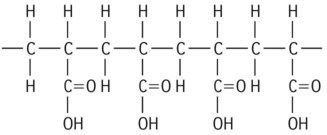
| Polyacrylic acid |
 |
 |
| Polymethylmethacrylate |
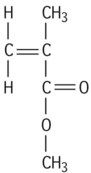 |
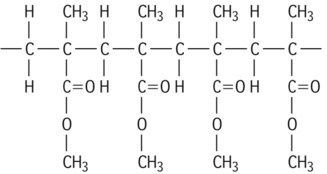 |
It should be noted that the chemical routes by which these different polymers are made are quite different, and that it is not a simple matter of modification to form one from the other. Each polymer has its own characteristic repeating unit, or ‘fingerprint’, and this unit is the basis for the widely differing properties of the polymers.
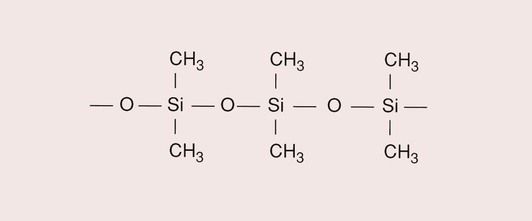
The most common polymers are those made from the organic compounds of carbon, but polymers can also be made from inorganic compounds, based on silica (SiO2).
Silicon, being four-valent like carbon, provides the opportunity to form the backbone for the polymer, together with oxygen. An example of a silicone polymer is polydimethylsiloxane (Figure 1.5.3).
When a polymer is formed from a single species of monomer, it is called a homopolymer; when different species are included, it is called a heteropolymer.
Mechanisms of polymerization
The monomers shown in Table 1.5.1 all have a double bond in common, which is opened up to allow the monomer to bond to a neighbouring monomer. This process of preparing polymers from monomers is called polymerization. There are two ways in which this may be achieved: addition and condensation.
Addition polymerization
Addition polymerization is defined as occurring when a reaction between two molecules (either the same to form a homopolymer, or dissimilar to form a heteropolymer) produces a larger molecule without the elimination of a smaller molecule (such as water).
This type of reaction takes place for vinyl compounds, which are reactive inorganic compounds containing carbon–carbon double bonds (see Table 1.5.1). The process of addition polymerization involves four stages to produce these polymers:
Activation
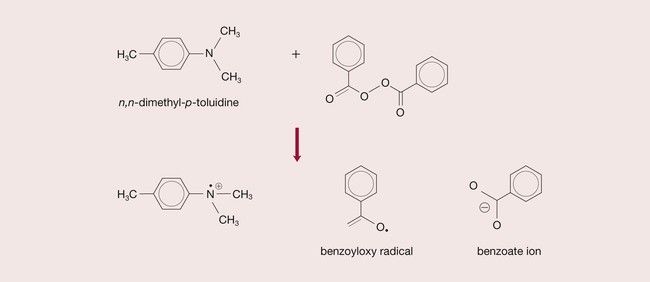
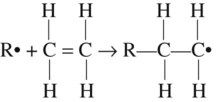
The polymerization of a vinyl compound requires the presence of free radicals (•). These are very reactive chemical species that have an odd (unpaired) electron. The process of producing free radicals is described as activation. Activation occurs, for instance, in the decomposition of a peroxide.
The peroxide commonly used in dental materials is benzoyl peroxide. Under appropriate conditions, a molecule of benzoyl peroxide can yield two free radicals:
C6H5COO −− OOCH5C6→2(C6H5COO•)
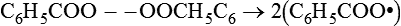
This in turn can decompose to form other free radicals:
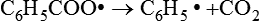
Such chemical species, known as initiators, are able to initiate vinyl polymerization, as described later, and are designated as R•.
Before initiation occurs, however, the benzoyl peroxide needs to be activated. This activation is achieved by the decomposition of the peroxide, due to the use of an activator, such as:
Other forms of free radical production include the use of ultraviolet light in conjunction with a benzoin methyl ether, and visible light with an α-diketone and an amine (see Chapter 2.2).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses