Atomic building blocks
Introduction
All materials are built up from atoms and molecules, so it is not really surprising that there is a close relationship between the atomic basis of a material and its properties. Important in this context are the nature of the atoms and the ways in which they are arranged. The atoms combine to determine the microstructure of the solid, and, as a consequence, determine its properties. Therefore, if we are to understand the properties of materials, we need to have an understanding of the way atoms can combine to make solids.
Joining atoms together
When two atoms are brought together, they may link to form a molecule; any bonds that form are called primary bonds. Alternatively, they may move apart and so retain their individual identity. Depending on the degree of interaction between the atoms, one of three states can form, these being gases, liquids or solids. These are referred to as the three main phases of matter, where a phase is defined as a structurally homogeneous part of the system and each phase will have its own distinct structure and associated properties. In the gaseous state there is little or no resistance to the relative movement of atoms or molecules, while in the liquid state the resistance to movement is considerably greater, but molecules can still flow past each other with great ease. In solids the movement of atoms and molecules is restricted to a local vibration, although some movement at the atomic level is possible through diffusion.
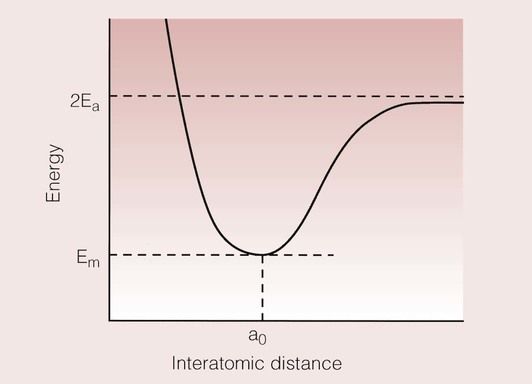
The controlling factor in bond formation is energy, and a bond will only form if it results in a lowering of the total energy of the atoms being joined. This means that the total energy of the molecule must be less than the sum of the energies of the separate atoms, irrespective of the type of bond being formed. A simple way of visualizing this is the energy-separation diagram, which considers what effect moving two atoms closer together will have on their total energy. A typical energy-separation curve is shown in Figure 1.2.1.
When the two atoms are far apart, the total energy is 2Ea, where Ea is the total energy of one atom. As they are brought closer together, the total energy begins to fall, until it reaches a minimum, Em, at a distance ao. Thereafter, as the atoms are brought more closely together, the total energy increases due to repulsion between their clouds of electrons. As the atoms are brought even closer together, their nuclei begin to repel each other as well, but such proximity is not usually achieved in normal circumstances. Thus, we have attraction at long range, and repulsion at short range.
The conditions under which two atoms will bond together depend on the atoms’ electron configurations, which completely determine their chemical reactivity. The more stable the electron configuration, the less reactive the atom; the extremes of stability are the ‘inert gases’, such as argon, helium and neon, which are almost totally non-reactive. Their near-inertness is caused by their having complete outermost electron orbitals, with no opportunity for more electrons to ‘join’ the atom, and no ‘spare’ or ‘loose’ electrons to leave the atom.
All atoms try to reach their lowest energy state, and this is tantamount to having a complete outermost electron orbital, as the inert gases have. The atoms of some elements have ‘gaps’ for electrons in their outermost orbits, whereas the atoms of other elements have ‘spare’ electrons in their outermost orbits. By combining with each other, these two different types of atoms can both achieve complete outermost orbitals. The formation of bonds, therefore, involves only the outermost valence electrons.
Types of primary bonds
There are three types of primary bond: covalent, ionic and metallic.
Covalent bonds
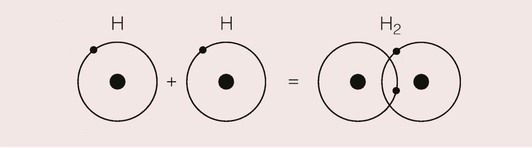
The covalent bond is the simplest and strongest bond, and arises when atoms share their electrons so that each electron shell achieves an inert gas structure. The formation of such a bond for two hydrogen atoms is shown in Figure 1.2.2.
As the two atoms approach one another and the orbitals of the electrons begin to overlap, a molecular orbital is formed where the two electrons are shared between the two nuclei. Since the electrons will spend most of their time in the region where the orbitals overlap, the bond is highly directional.
Ionic bonds
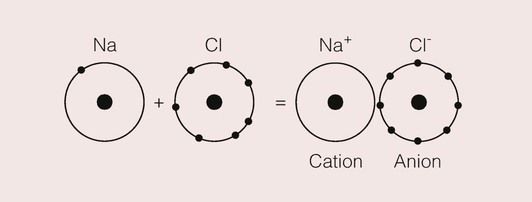
An atom such as sodium would like to lose its single valence electron, as this would give it a configuration similar to that of neon. Naturally, it cannot do so unless there is another atom nearby which will readily accept the electron.
Elements, which can attain an inert gas structure by acquiring a single extra electron, are fluorine, chlorine, bromine and iodine, collectively known as the halogens. Thus, if a sodium and a chlorine atom are allowed to interact, there is a complete transfer of the valence electron from the sodium atom to the chlorine atom. Both attain an inert gas structure, with sodium having a positive charge due to loss of a negative electron, and chlorine a negative charge due to its acquisition of the extra electron. These two ions will be attracted to one another because of their opposite electrical charges, and there is a reduction in the total energy of the pair as they approach. This is shown in the model in Figure 1.2.3; such bonds are called ionic bonds.
An important difference between the covalent bond and the ionic bond is that the latter is not directional. This is because ionic bonds are a result of the electrostatic fields that surround ions, and these fields will interact with any other ions in the vicinity.
Metallic bonds
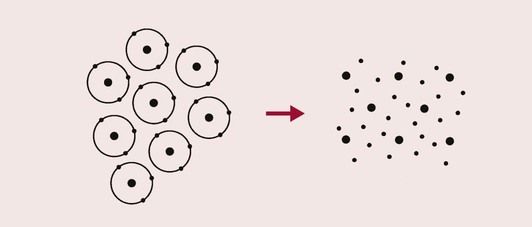
The third primary bond is the metallic bond. It occurs when there is a large aggregate of atoms, usually in a solid, which readily give up the electrons in their valence shells. In such a situation, the electrons can move about quite freely through the solid, spending their time moving from atom to atom. The electron orbitals in the metallic bond have a lower energy than the electron orbitals of the individual atoms. This is because the valence electrons are always closer to one or other nucleus than would be the case in an isolated atom. A cloud of electrons, as shown in Figure 1.2.4, surrounds the atoms. Like the ionic bond, this bond is non-directional.
Bond energies
An important feature of a bond is the bond energy. This is the amount of energy that has to be supplied to separate the two atoms, and is equal to 2Ea − Em, as defined in Figure 1.2.1. Typical bond energies for each of the three types of bond are given in Table 1.2.1.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses