Surgical Complications in Implant Placement
Paul B. Greenawalt
Private Practice in Oral and Maxillofacial Surgery, Poulsbo, Washington, USA
Dental implants have become a widely used component of comprehensive treatment planning to restore partially and completely edentulous patients.1 In spite of their documented long‐term survival rates,2 there has been a tendency to underappreciate the incidence and complexity of restorative and surgical complications. When planning an implant‐borne prosthesis, it is critical to develop a restoratively driven blueprint in order to identify the optimal location, diameter, and angulation of the implant(s). Surgical templates may be constructed from diagnostic wax‐ups, generated from three‐dimensional (3‐D) imaging such as cone‐beam computed tomography (CBCT), or from a model fabricated by a CAD/CAM machine. The purpose of this guide is to communicate the pertinent spatial requirements and restrictions for implant placement to support a functional and esthetic restoration. However, precise execution of this task continues to be challenging due to potential mishaps in the light of the many variables that must be taken into account.
Avoidance of surgical complications can be enhanced by a thorough knowledge of local and regional anatomy, pretreatment imaging, and sound surgical principles. Untoward results may be related to host limitations, anatomic constraints, or execution of implant placement. As with any medical implantable device there are associated risks and ensuing problems. This chapter will focus on complications associated with implant surgery as well as their prevention and management.
Complications during execution
Neurologic disturbances
To discuss nerve injuries, and their sequelae, we shall first review classification of injuries. Seddon,3 in 1942, described injuries as belonging to one of three classifications: neurapraxia, axonotmesis, and neurotmesis. Sunderland,4 in 1978, then described injuries and classified them from 1 to 5. MacKinnon/Dellon5 (M/D), in 1988, further described a sixth classification that is a neuroma‐in‐continuity. The purpose of classifying injuries to nerves is to better prognosticate the likelihood of recovery, and to what degree (Table 7.1).
Table 7.1 Classifications of nerve injuries.
| Sunderland | Seddon | Description of Injury | Recovery period | |
| Least severe | I | Neurapraxia | Conduction block, no Wallerian degeneration | <3 months |
| II | Axonotmesis | Axonal discontinuity, nerve remains intact | 25 mm (1 inch)/month | |
| III | Extensive scarring of the endoneurium hinders axon regeneration | <25 mm (1 inch)/month | ||
| IV | Nerve still in‐continuity, scar blocks nerve regeneration | Surgical intervention required to remove scar tissue | ||
| Most severe | V | Neurotmesis | Rupture of nerve | Recovery requires surgery |
Neurapraxia (Sunderland 1, M/D 1) is characterized by a temporary loss of sensation that returns in days to weeks. The nerve trunk is continuous, as is the axon, however there is demyelination or loss of the myelin sheath. Axonotmesis (Sunderland 2 or 3, M/D 2 or 3) is also characterized as loss of sensation. With class 2 injuries, sensation typically returns in 3–6 months whereas type 3 injuries have incomplete resolution in 3–6 months. The nerve trunk is continuous, however the axon is not and there is demylelination. There is progressively more axonal damage in class 3 as compared to class 2. Neurotmesis (Sunderland 4 or 5, M/D 4, 5, or 6) is characterized by incomplete resolution of symptoms after 3–6 months to no sensation after 3–6 months. Class 6, or the neuroma‐in‐continuity, has a mixed response after 3–6 months and slightly better long‐term prognosis compared to the class 4 or 5 injury. With class 4, 5, and 6 injuries there is nerve trunk disruption, partial to complete, axons are not continuous, with accompanying demyelination.
Nerve injuries may also be classified in a qualitative fashion. This is somewhat easier to relate to patient symptoms and clinical presentation of the injury but is too broad and subjective to quantify the injury. Nevertheless, it is helpful when discussing a nerve injury with a patient. Anesthesia, or adding the qualifier “total” anesthesia, is a term used to describe a complete loss of sensation. In this scenario, noxious stimuli elicit no response (Figure 7.1). More frequently, patients describe an area of profound numbness and also may have a diminished response to noxious stimuli. For example, when testing for perception of sharp versus dull stimulus, patients with anesthesia will note no difference, while partial anesthesia may allow for a diminished ability to discern. They may be able to identify that there is a difference but they simply are unable to replicate sensations on the unaffected side. Paresthesia describes altered sensation, often described as tingling, burning, or pinpricks. Dysesthesia, however, is a condition whereby ordinary stimuli are perceived as noxious and unpleasant. Paresthesia is seen with minor or incomplete injuries, particularly immediately following surgery. Anesthesia typically results from a more severe injury, including transection. Dysesthesia is frequently a late‐onset symptom that is the result of scarring, a neuroma, or neuroma‐in‐continuity.
Figure 7.1 Assessing anesthesia.
A simple assessment kit (Figure 7.2) may be assembled and kept on hand to evaluate a patient’s sensory disturbance if that should occur. Wooden stick cotton tip applicators can be used to evaluate light touch and direction, sharp versus dull, and cold versus not cold. The cotton is pulled into a wisp and, with the patients eyes closed, they are asked to point in the direction that they perceive touch and direction. The nonaffected side may be used as the “control”. Soaking the cotton tip in ethyl chloride will render it cold. Again with eyes closed the cold cotton tip and an untreated tip are applied randomly to the affected and nonaffected sides. Sharp versus dull is tested with a broken wooden stick cotton tip applicator and the cotton tip itself. If desired, two‐point discrimination with ordinary calipers can be used to document the sensory disturbance.
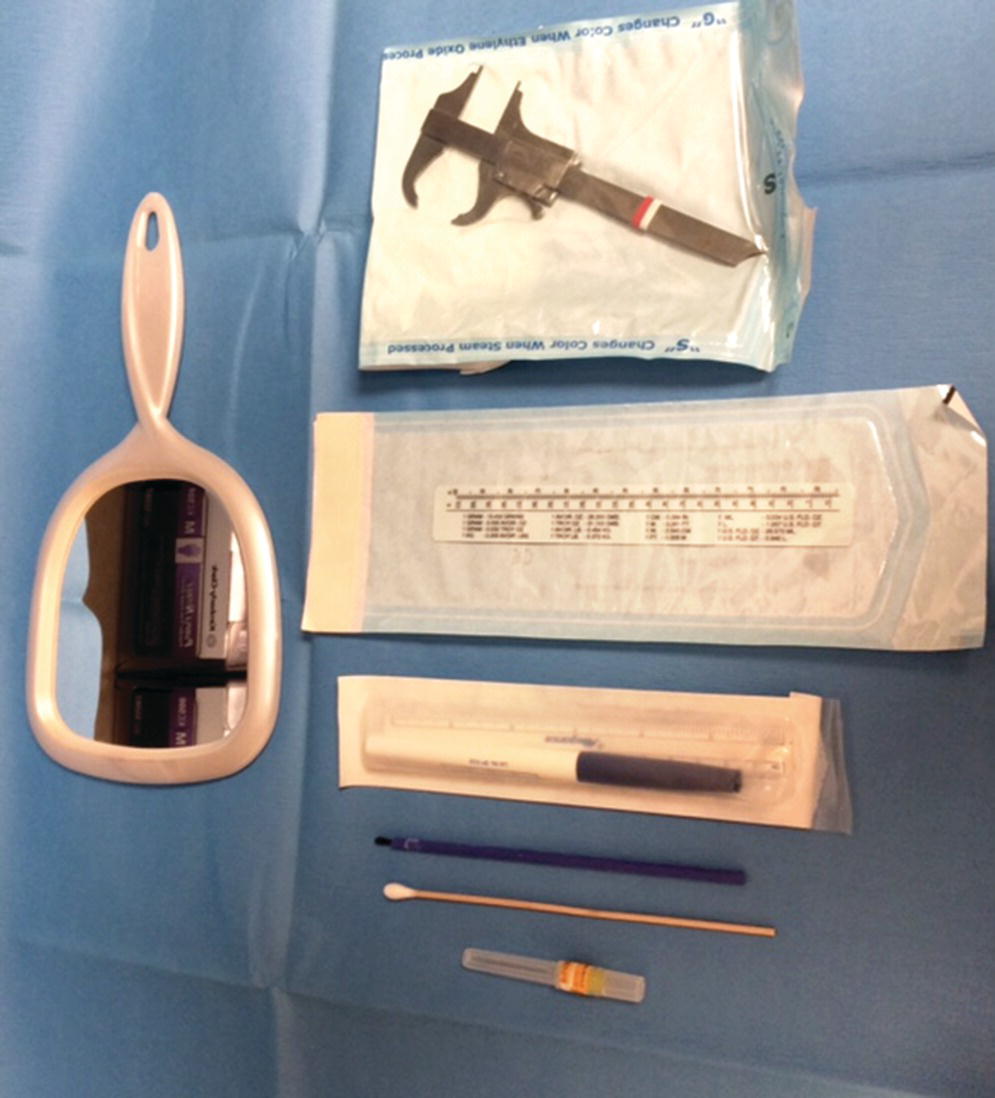
Figure 7.2 Kit for evaluating nerve injuries.
In implant dentistry, the nerve most at risk is the inferior alveolar nerve. This can occur with over‐instrumentation during osteotomy or overseating the implant, thereby compressing the canal from above. Over‐instrumentation can occur in the mandible when firm pressure is required to penetrate the dense cortical plate. When the cortex is breached, the cancellous bone beneath provides little resistance and inadvertent overpenetration can ensue. Drill guards, intraoperative radiographs, and surgical restraint can all help to reduce this risk. Additionally, measuring the twist drill prior to use will confirm the accuracy of the depth markings relative to the actual implant length. Nerve injuries may be avoided with meticulous preparation, imaging, and execution. Treatment planning or mock surgery using the radiographic image with correction for image magnification is very helpful in avoiding this misadventure. A minimum distance of 2 mm must be maintained between the implant and the nerve canal.6 The mental foramen, the point of exit for the inferior alveolar nerve from its bony canal, poses particular anatomic challenges. The nerve will typically form an anterior loop, or genu, in the body of the mandible before exiting through the mental foramen. The loop is variable but has been reported to be between 1 and at least 5 mm anterior to the foramen. A survey of dried mandibles by Misch and Crawford7 reported lengths of the loop to be up to 5 mm. To safely place an implant, the implant body needs to be 2 mm from the nerve. Considering the greatest extent of the loop, 5 mm, plus the 2 mm safety margin, the implant should be no closer than 7 mm from the mental foramen. It is with this guideline that Bedrossian6 creates a path of insertion for an angled posterior implant that avoids the nerve by a minimum of 2 mm. This is achieved by placing the inferior‐most point of the implant 7 mm anterior to the mental foramen (Figure 7.3).
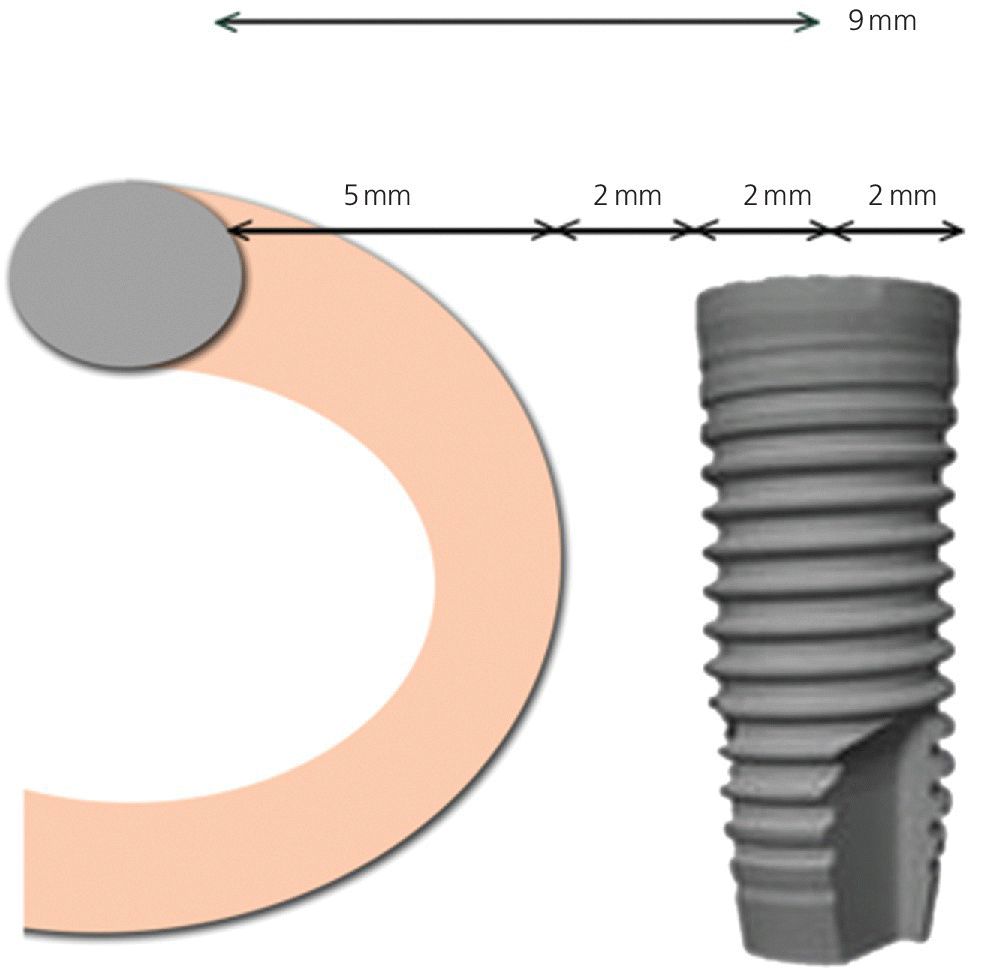
Figure 7.3 Distance from anterior loop of the inferior alveolar nerve, measuring for safe distance from the mental foramen.
Nerve injury can also occur through poor flap design, poor flap development, and excessive retraction. Failing to account for the mental nerve and its branches as they course through soft tissue is but one way of securing a nerve injury. As the inferior alveolar nerve travels through the mandible from the mandibular foramen to the mental foramen it also trends outward from the lingual cortex to the internal aspect of the buccal cortex.7 Three‐dimensional imaging can accurately identify the inferior alveolar canal as it wends its way from proximal to distal. Treatment‐planning software can also aid in identifying potential sites for nerve injury during implant placement. A partial‐thickness flap rather than a full‐thickness flap can directly traumatize a nerve. Meticulous attention to develop a subperiosteal plane for elevation will help to protect the nerve as it courses through soft tissue. The periosteum is tethered to the mental nerve as it exits the mental foramen. If an extensive flap must be elevated in this area, careful dissection of periosteum from the nerve may be necessary to prevent traction on the nerve. Flapless procedures also carry inherent risk due to lack of direct visualization and potentially “losing one’s way”. A sound understanding of anatomy coupled with 3‐D imaging can reduce this risk.
Direct trauma from injection of local anesthesia as well as chemical trauma from the anesthetic agent itself may also play a role in the constellation of causative factors for nerve injuries.8,9 An increasing percentage of local anesthetic carries with it additional risk for chemical trauma. Hillerup et al.10 in 2011 concluded that local anesthetic in a 4% solution posed a statistically significant greater risk of nerve injury than a lower concentration, such as 2%.
Management of nerve injuries
If, on initial examination of the patient postoperatively, there is altered sensation, a critical examination of radiographs is essential to determine if the implant violated the canal. If the apex of the implant is within the 2 mm “safety” margin, then the surgeon must consider that the implant is compressing the overlying bone and thereby the nerve. If feasible, backing the implant out a few turns may alleviate the compression. This, however, may impact initial stability and/or the position of the head of the implant relative to crestal bone. Inflammation may often be a component of nerve injury in addition to the physical injury. Steroid therapy with or without concurrent nonsteroidal anti‐inflammatory drug (NSAID) therapy will mitigate the inflammatory response. Ibuprofen, 800 mg three times per day for a minimum of 14 days, is a typical NSAID regimen. High‐dose prednisone in a rapid taper has been shown to greatly suppress the inflammatory response.11,12 A regimen that has been proffered is 60 mg for 3 days, 40 mg for 3 days, and finally 20 mg for 3 days. Second‐ and third‐generation anticonvulsants such as oxcarbapine (Trileptal), zonisamide (Zonegram), topirimate (Topamax), levetiracetam (Keppra), and lamotrigine (Lamictal), have shown promise, possibly due to their gamma amino butyric acid (GABA) effects as a neuroinhibitor (Figure 7.4).13 Earlier‐generation anticonvulsants, such as carbamazapine (Tegretol), have also been prescribed.
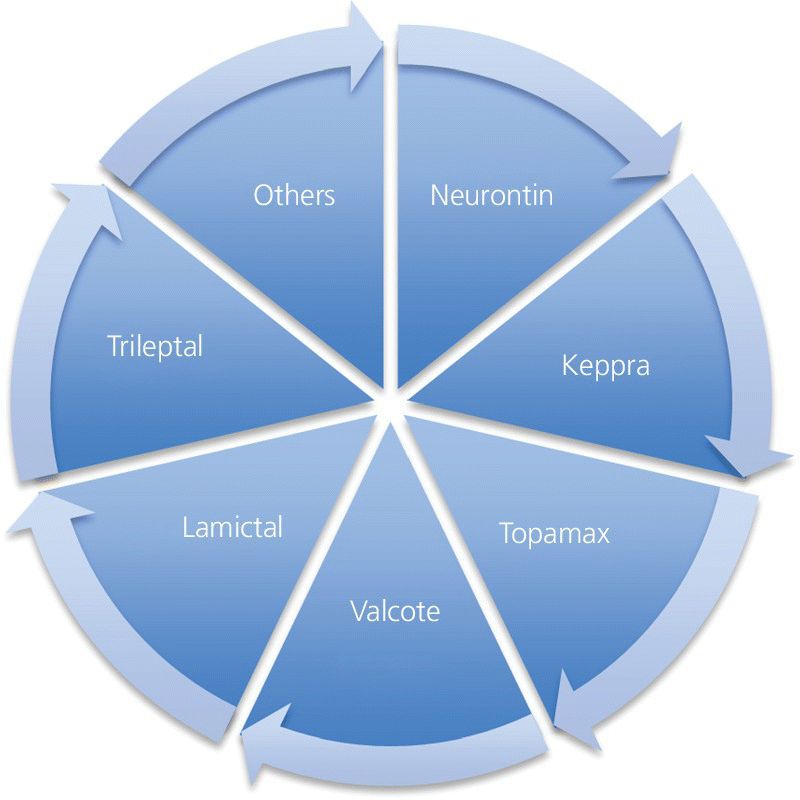
Figure 7.4 Drugs for medical management of nerve injuries.
Antiviral drugs and hyperbaric oxygen (HBO) are also strategies effective in reducing endoneurial edema. Beyond these strategies, B‐complex vitamins may also be of benefit. If improvement is noted with medical management, the surgeon may elect to continue the therapy for an additional 3 weeks.
If anesthesia is persistent, or if, after 16 weeks, dysesthesia persists, the patient should be referred to a microneurosurgeon for consultation. It has been suggested that if repair is performed before Wallerian degeneration of the injured nerve takes place, patients report favorable responses.14 Wallerian degeneration, as described by Augustus Volney Waller,15 in 1850, is the degeneration of the nerve, particularly the Schwann cells distal to the injury. Typically, the distal nerve trunk has not reached its maximum degeneration for 4–6 months after nerve injury. Sensation may not be restored but subjective improvement is often perceived after degeneration is complete. This degeneration is a slow process, and the reconstructive surgeon may elect to delay the repair or repair the nerve immediately.
Floor of mouth injuries
The contents of the floor of the mouth are both numerous and varied. The lingual artery, its branches, and bone perforations will be discussed later in this chapter. Other structures include muscles, salivary glands and their ducts/orifices, as well as nerves.
Muscles in the floor of the mouth not only provide movement but also act as a hammock delineating superficial and deeper spaces. The mylohyoid muscle originates on the lingual aspect of the mandible at the mylohyoid ridge and inserts on the superior aspect of the hyoid bone. Its function is to elevate the contents of the floor of mouth thereby aiding in mastication and swallowing. The ridge is closest to the alveolar crest in the posterior and deepest in the anterior. The mylohyoid muscle serves as an anatomic barrier between the sublingual space and the submandibular space. Violation of it allows for introduction of materials such as bacteria, saliva, and bone into deeper spaces, perhaps raising the risk of a deep space infection. The genioglossus muscles are a paired set that originate on the genial tubercles which arise on the lingual aspect of the mandible, deep to the alveolus. They insert on the deep oropharynx mucosa, lingual fascia, and epiglottis. Their function is to depress and protrude the tongue. In the severely resorbed mandible, the tubercles are often directly below the mucosa. Intrusion into the genioglossus muscle provides the possibility for profound bleeding, introduction of bacteria into deep spaces, and potential airway difficulty.
The tongue itself is also subject to injury, though more likely from a high‐speed rotary instrument or disc. Moreover, if a limited alveoplasty is being performed to create adequate width for the implant head then it too can engage the errant tongue, even with an alert surgeon and assistant. Immediate exploration of any injury is vital so as to determine the extent of the injury and initiate a repair if indicated.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


