Tumors of the Salivary Glands
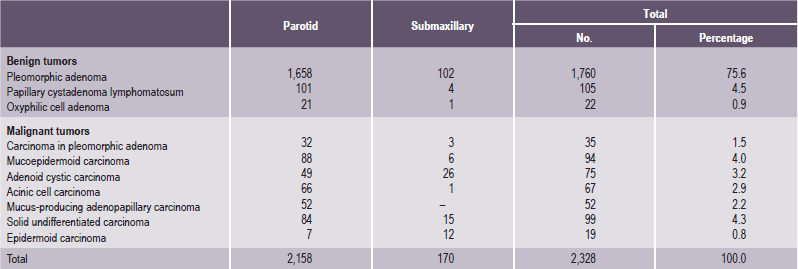
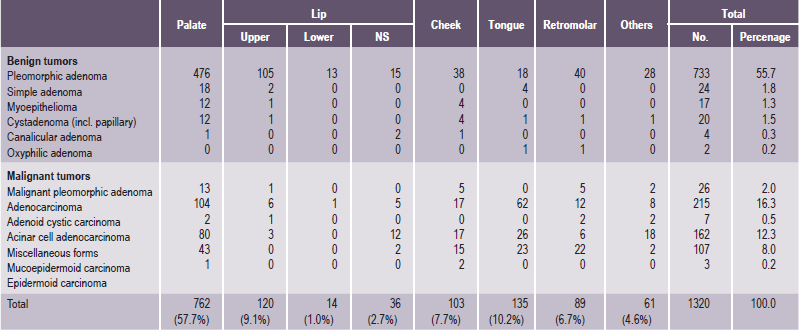
It is important to recognize that neoplasms may arise not only from the major salivary glands, but also from any of the numerous, diffuse, intraoral accessory salivary glands. Thus one may expect to see tumors originating from the glands in the lip, palate, tongue, buccal mucosa, floor of the mouth and retromolar area. Salivary gland tumors are much more common on the hard palate than on the soft, probably because there are a greater number of gland aggregates on the hard palate than on the soft palate. With only occasional exceptions, any type of tumor which occurs in a major salivary gland may also arise in an intraoral accessory gland. Thus, in the following discussion, the general features described under each tumor will hold true for both major and minor salivary gland lesions. There appear to be no truly specific, recognized tumors native only to the intraoral glands. Annual incidence of salivary gland tumors around the world is stated to be 1–6.5 cases per 100,000 people. Most studies have shown that minor salivary gland tumors are more common in females than males, with a ratio range from 1.2 : 1–1.9 : 1. Eneroth has presented data on over 2,300 tumors of the major salivary glands (Table 3-1), while a complete review of the intraoral minor salivary gland tumors has been published by Chaudhry and his coworkers, with much valuable information obtained from the analysis of over 1,300 cases (Table 3-2).
Benign tumors of the salivary gland need no treatment other than surgical removal. Malignant tumors, on the other hand, may require radiation or chemotherapy or both after surgery. Surgery for salivary gland tumors removes all or most of the affected glands, not just the tumor. When performing surgery on these glands, great care is taken to identify and protect the nerves that pass through or near these glands and supply the muscles of the face, mouth and tongue. These nerves are stretched during surgery, which results in a temporary weakness on part of the face in up to 15% of patients. This weakness is temporary and usually disappears in one to three months. Occasionally, malignant tumors invade the nerves that supply part or all of the muscles of the face. When this occurs, a portion of the nerve is surgically removed with the tumor, and a nerve graft is used to rebuild the nerve.
Benign Tumors of the Salivary Glands
Pleomorphic Adenoma (Mixed tumor)
Pleomorphic adenoma is a benign neoplasm consisting of cells exhibiting the ability to differentiate to epithelial (ductal and nonductal) cells and mesenchymal (chondroid, myxoid and osseous) cells. This tumor has been referred to by a great variety of names through the years (e.g. mixed tumor, enclavoma, branchioma, endothelioma, enchondroma), but the term ‘pleomorphic adenoma’ suggested by Willis characterizes closely the unusual histologic pattern of the lesion. It is almost universally agreed that this tumor is not a ‘mixed’ tumor in the true sense of being teratomatous or derived from more than one primary tissue. Its morphologic complexity is the result of the differentiation of the tumor cells, and the fibrous, hyalinized, myxoid, chondroid and even osseous areas are the result of metaplasia or are actually products of the tumor cells per se. Pleomorphic adenoma is the most common salivary gland tumor (Table 3-3).
Table 3-3
Histological classification of salivary gland tumors (WHO 1991)
1. Adenomas
• Pleomorphic adenoma
• Myoepithelioma (myoepithelial adenoma)
• Basal cell adenoma
• Warthin’s tumor (adenolymphoma)
• Oncocytoma (oncocytic adenoma)
• Canalicular adenoma
• Sebaceous adenoma
• Ductal papilloma
▫ Inverted ductal papilloma
▫ Intraductal papilloma
▫ Sialadenoma papilliferum
• Cystadenoma
▫ Papillary cystadenoma
▫ Mucinous cystadenoma
2. Carcinomas
• Acinic cell carcinoma
• Mucoepidermoid carcinoma
• Adenoid cystic carcinoma
• Polymorphous low grade adenocarcinoma (terminal duct adenocarcinoma)
• Epithelial-myoepithelial carcinoma
• Basal cell adenocarcinoma
• Sebaceous carcinoma
• Papillary cystadenocarcinoma
• Mucinous adenocarcinoma
• Oncocytic carcinoma
• Salivary duct carcinoma
• Adenocarcinoma
• Malignant myoepithelioma (myoepithelial carcinoma)
• Carcinoma in pleomorphic adenoma (malignant mixed tumor)
• Squamous cell carcinoma
• Small cell carcinoma
• Undifferentiated carcinoma
• Other carcinomas
3. Nonepithelial tumors
4. Malignant lymphomas
5. Secondary tumors
6. Unclassified tumors
7. Tumor like lesions
• Sialadenosis
• Oncocytosis
• Necrotizing sialometaplasia (salivary gland infarction)
• Benign lymphoepithelial lesion
• Salivary gland cysts
• Chronic sclerosing sialadenitis of submandibular gland (Küttner tumor)
• Cystic lymphoid hyperplasia in AIDS
Histogenesis
Numerous theories have been advanced in explaining the histogenesis of this bizarre tumor. Currently, these center around the myoepithelial cell and a reserve cell in the intercalated duct. Ultrastru ctural studies have confirmed the presence of both ductal and myoepithelial cells in pleomorphic adenomas. It follows that possibly either or both may play active roles in the histogenesis of the tumor. Hubner and his associates have postulated that the myoepithelial cell is responsible for the morphologic diversity of the tumor, including the production of the fibrous, mucinous, chondroid and osseous areas. Regezi and Batsakis postulated that the intercalated duct reserve cell can differentiate into ductal and myoepithelial cells and the latter, in turn, can undergo mesenchymal metaplasia, since they inherently have smooth muscle like properties. Further differentiation into other mesenchymal cells then can occur. Batsakis has discussed salivary gland tumorigenesis, and while still implicating the intercalated duct reserve cell as the histogenetic precursor of the pleomorphic adenoma, stated that the role of the myoepithelial cell is still uncertain and that it may be either an active or passive participant histogenetically. Finally, Dardick and his associates have questioned the role of both ductal reserve and myoepithelial cells. They state that a neoplastically altered epithelial cell with the potential for multidirectional differentiation may be histogenetically responsible for the pleomorphic adenoma.
Clinical Features
Pleomorphic adenoma is the most common tumor of salivary glands. The parotid gland is the most common site of the pleomorphic adenoma; 90% of a group of nearly 1,900 such tumors reported by Eneroth. It may occur in any of the major glands or in the widely distributed intraoral accessory salivary glands; however its occurrence in the sublingual gland is rare. In the parotid this tumor most often presents in the lower pole of the superficial lobe of the gland, about 10% of the tumors arise in the deeper portions of the gland. Approximately 8% of pleomorphic adenomas involve the minor salivary glands, the palate is the most common site (60–65%) of minor salivary gland involvement. It occurs more frequently in females than in males, the ratio approximating 6 : 4. The majority of the lesions are found in patients in the fourth to sixth decades with the average age of occurrence of about 43 years, but they are also relatively common in young adults and have been known to occur in children. The clinical behavior of this tumor in children is similar to that in adults (Table 3-4).
Table 3-4
TNM/AJCC 1997 staging (clinical staging)
TX: primary tumor cannot be assessed
• T0: No evidence of primary tumor
• T1: Tumor 2 cm or less in greatest dimension without extraparenchymal extension*
• T2: Tumor > 2 cm but < 4 cm in greatest dimension without extraparenchymal extension*
• T3: Tumor having extraparenchymal extension* without seventh nerve involvement and/or more than 4 cm but no more than 6 cm in greatest dimension
• T4: Tumor invades base of skull, seventh nerve, and/or exceeds 6 cm in greatest Dimension
• N0: No regional node metastasis
• Nx: Regional nodes cannot be assessed
• N1: Single ipsilateral node, < 3 cm
• N2a: Single ipsilateral node, > 3 cm and < 6 cm
• N2b: Multiple ipsilateral nodes, < 6 cm
• N2c: Contralateral or bilateral nodes, < 6 cm
• N3: Node > 6 cm
• MO: No distant metastasis
• Mx: Metastasis cannot be assessed
Minor salivary gland tumors are staged according to their site of origin.
*Extraparenchymal extension is clinical or macroscopic evidence of invasion of skin, soft tissues, bone or nerve.
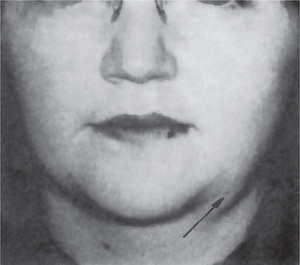
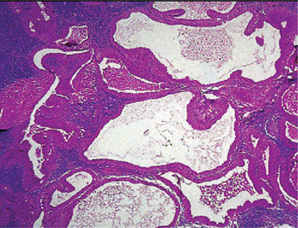
The history presented by the patient is usually that of a small, painless, quiescent nodule which slowly begins to increase in size, sometimes showing intermittent growth (Fig. 3-1). The pleomorphic adenoma, particularly of the parotid gland, is typically a lesion that does not show fixation either to the deeper tissues or to the overlying skin (Fig. 3-2 A). It is usually an irregular nodular lesion which is firm in consistency, although areas of cystic degeneration may sometimes be palpated if they are superficial. The skin seldom ulcerates even though these tumors may reach a fantastic size, lesions having been recorded which weighed several kilograms (Fig. 3-2 B). Pain is not a common symptom of the pleomorphic adenoma, but local discomfort is frequently present. Facial nerve involvement manifested by facial paralysis is rare.
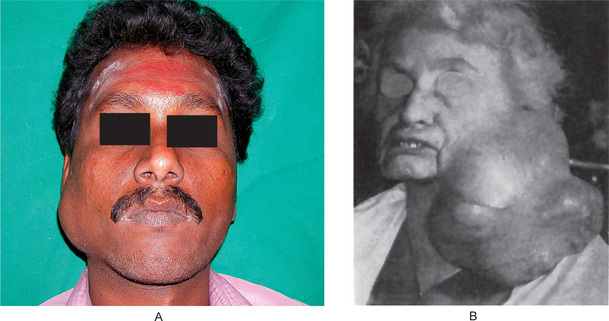
Figure 3-2 Pleomorphic adenoma of parotid gland.
(A) Typical appearance of pleomorphic adenoma of parotid gland. (B) The lesion here is used not to illustrate the usual clinical appearance of a pleomorphic adenoma of the parotid gland, but to demonstrate the size which these tumors may attain. This lesion was present for eighteen years A, Courtesy of Dr Neelakandan RS, Department of Oral and Maxillofacial Surgery, Meenakshi Ammal Dental College, Chennai.
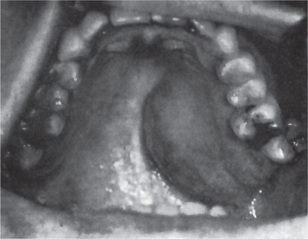
The pleomorphic adenoma of intraoral accessory glands seldom is allowed to attain a size greater than 1–2 cm in diameter. Because this tumor causes the patient difficulties in mastication, talking and breathing, it is detected and treated earlier than tumors of the major glands. The palatal glands are frequently the site of origin of tumors of this type (Fig. 3-3), as are the glands of the lip (Fig. 3-4) and occasionally other sites. Except for size, the intraoral tumor does not differ remarkably from its counterpart in a major gland. The palatal pleomorphic adenoma may appear fixed to the underlying bone, but is not invasive. In other sites the tumor is usually freely movable and easily palpated. Recurrent lesions, however, occur as multiple nodules and are less mobile than the original tumor (Table 3-5).
Histologic Features
Morphologic diversity is the most characteristic feature of this neoplasm. Microscopically, benign mixed tumors are characterized by variable, diverse, structural histologic patterns, seldom do individual cases resemble each other, considerable variation is also seen within a single tumor. Pleomorphic adenomas demonstrate combinations of glandular epithelium and mesenchyme like tissue and the proportion of each component varies widely among individual tumors. Foote and Frazell (1954) categorized the tumor into the following types:
Table 3-6
Typical features of benign and malignant salivary gland tumors
| Benign salivary gland tumors | Malignant salivary gland tumors |
| • Slow growing | • Sometimes fast growing |
| • Soft or rubbery consistency | • Sometimes hard consistency |
| • 85% of parotid tumors are benign | • 45% of minor glands are malignant |
| • Do not ulcerate | • May ulcerate and invade bone |
| • No associated nerve signs | • May cause cranial nerve palsies (e.g. parotid tumor causes facial palsy, adenoid cystic carcinoma can cause multiple nerve lesions especially of lingual, facial or hypoglossal nerves) |
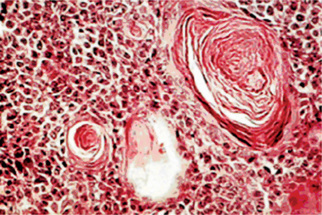
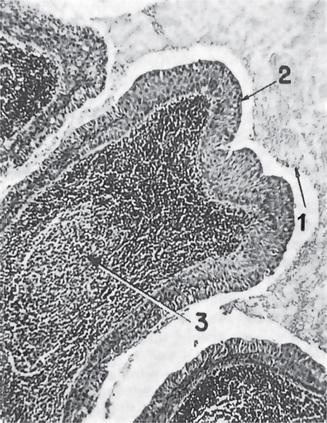
The epithelial component forms ducts and small cysts that may contain an eosinophilic coagulum, the epithelium may also occur as small cellular nests, sheets of cells, anastomosing cords and foci of keratinizing squamous or spindle cells. Myoepithelial cells are a major component of pleomorphic adenoma. They have a variable morphology, sometimes appearing as angular or spindled, while some cells are more rounded with eccentric nuclei and hyalinized eosinophilic cytoplasm resembling plasma cells (earlier referred to as hyaline cells) (Fig. 3-5). Myoepithelial-cells are also responsible for the characteristic mesenchyme like changes; these changes are brought about by extensive accumulation of mucoid material around individual myoepithelial cells giving a myxoid appearance. Vacuolar degeneration of these myoepithelial cells then results in a cartilaginous appearance (Fig. 3-6). Foci of hyalinization, bone and even fat can be noted in the connective tissue stroma of many tumors. When the pleomorphic pattern of the stroma is absent, and the tumor is highly cellular, it is often referred to as a ‘cellular adenoma’. When myoepithelial proliferation predominates, the diagnosis of ‘myoepithelioma’ (q.v.) is generally made.
Myoepithelioma: (Myoepithelial adenoma)
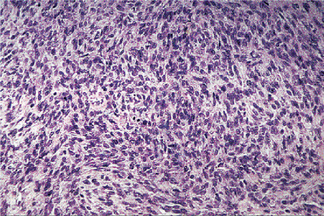
Histologic Features
The tumor is composed exclusively, or almost exclusively, of neoplastic myoepithelial cells. The neoplastic cells are predominantly spindle-shaped or plasma-cytoid. Epithelioid or clear cells may also be present (Figs. 3-7, 3-8). Either a single cell type predominates in a tumor or there may be a combination of cell types. The tumor is often difficult to diagnose definitively at the light microscopic level. Myoep-ithelioma does not contain the characteristic chondromyxoid stroma of pleomorphic adenoma. Myoepithelioma consisting predominantly of spindle cells tends to be more cellular than the tumor consisting of predominantly plasmacytoid cells. Definitive diagnosis lies in the ultrastructural identification of myoepithelial cells. The myoepithelial cell exhibits a basal lamina and fine intracytoplasmic myofilaments. Desmosomes are encountered between adjacent cells.
Basal Cell Adenoma
Histologic Features
This pattern exhibits multiple small, round duct like structures. These tubules are lined by two distinct layers of cells, with inner cuboidal ductal cells surrounded by an outer layer of basaloid cells. The tubular variant is the least common; however, tubule formation either alone or with basal cell masses, can be found in most basal cell adenomas, at least focally (Fig. 3-9).
This subtype has the same cytologic features as the solid type, but the epithelial islands are narrower and cord like and are interconnected with one another, producing a reticular pattern (Fig. 3-10).
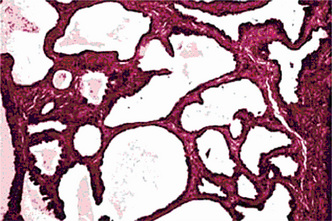
Warthin’s Tumor: (Papillary cystadenoma lymphomatosum, adenolymphoma)
Warthin’s tumor is the second most common tumor in the salivary glands. This tumor was first recognized by Albrecht in 1910 (quoted by Ellis and Auclair 1991) and later described by Warthin in 1929. This unusual type of salivary gland tumor occurs almost exclusively in the parotid gland, although occasional cases have been reported in the submaxillary gland. The intraoral accessory salivary glands are rarely affected.
Histologic Features
This tumor is made up of two histologic components: epithelial and lymphoid tissue. As the name would indicate, the lesion is essentially an adenoma exhibiting cyst formation, with papillary projections into the cystic spaces and a lymphoid matrix showing germinal centers (Fig. 3-11). The cysts are lined by papillary proliferations of bilayered oncocytic epithelium. The inner layer cells are tall columnar with finely granular and eosinophilic cytoplasm due to presence of mitochondria and slightly hyperchromatic nuclei. The outer layer cells are oncocytic triangular and occasionally fusiform basaloid cells. Focal areas of squamous metaplasia and mucous cell prosoplasia may be seen. There is frequently an eosinophilic coagulum present within the cystic spaces, which appears as a chocolate-colored fluid in the gross specimen. The abundant lymphoid component may represent the normal lymphoid tissue of the lymph node within which the tumor developed or it may actually represent a reactive cellular infiltrate which involves both humoral and cell mediated mechanisms (Fig. 3-12).
Oncocytoma: (Oncocytic adenoma, oxyphilic adenoma, acidophilic adenoma)
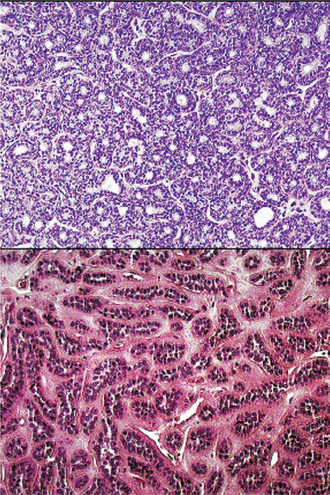
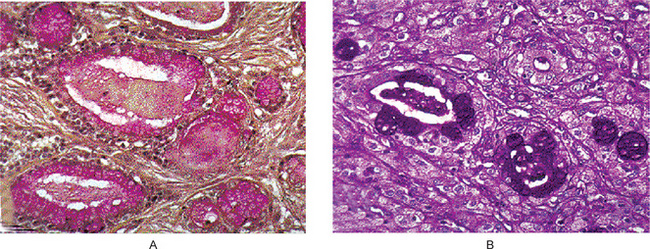
Oncocytoma is a rare benign tumor composed of oncocytes with granular eosinophilic cytoplasm and a large number of atypical mitochondria. This rare salivary gland tumor is a small benign lesion, which usually occurs in the parotid gland. Except that it does not generally attain any great size, it does not differ in its clinical characteristics from other benign salivary gland tumors. For this reason, a clinical diagnosis is difficult if not impossible to establish. The name ‘oncocytoma’ is derived from the resemblance of these tumor cells to apparently normal cells which have been termed ‘oncocytes’ and which are found in a great number of locations, including the salivary glands, respiratory tract, breast, thyroid, pancreas, parathyroid, pituitary, testicle, fallopian tube, liver and stomach. These cells are predominantly seen in duct linings of glands in elderly persons, but little is actually known of their mode of development or significance (Fig. 3-13). Electron microscopic studies have shown that the cytoplasm of the oncocyte is choked with mitochondria. Ionizing radiation is the predisposing condition.
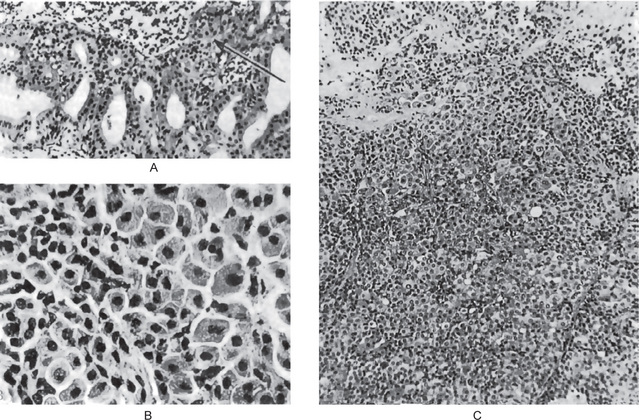
Figure 3-13 Oxyphilic adenoma.
(A) Normal oncocytes in accessory salivary gland ducts of elderly patient. (B) High-power photomicrograph. (C) Low-power photomicrograph of oncocytoma.
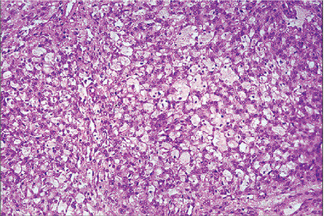
Histologic Features
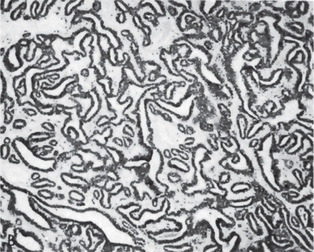
The oxyphilic adenoma is characterized microscopically by large cells which have an eosinophilic cytoplasm and distinct cell membrane and which tend to be arranged in narrow rows or cords (Fig. 3-13). The oncocytes are arranged in sheets or nests and cords, which form alveolar or organoid pattern. Some degree of cellular atypia, nuclear hyperchromatism and pleomorphism is accepted as compatible with benignancy in oncocytoma. These cells, exhibiting few mitotic figures, are closely packed, and there is little supportive stroma (Fig. 3-14). Lymphoid tissue is frequently present, but does not appear to be an integral part of the lesion. Ultrastructural studies of parotid oncocytomas by Tandler and associates and Kay and Still have shown that the cells are engorged with enlarged and morphologically altered mitochondria.
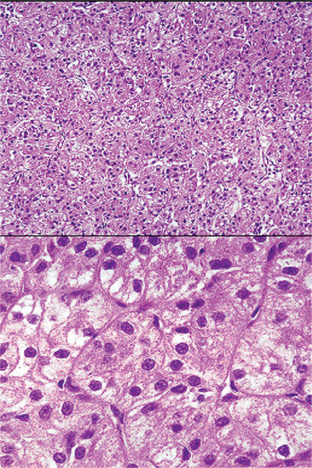
Figure 3-14 Oncocytoma alveolar pattern.
Illustrated by clusters of oncocytes that are supported by thin, fibrous connective tissue septa and small blood vessels. Note oncocytes have clear cytoplasm that is interspersed with cytoplasmic granularity.
A variant of the oxyphilic adenoma is sometimes seen in intraoral salivary glands, particularly in the buccal mucosa and upper lip. This has been termed, an oncocytic cystadenoma since it is a tumor like nodule composed chiefly of numerous dilated duct like or cyst like structures lined with oncocytes. In recurrent tumors, there may be marked clear cell change and these tumors may be referred to as clear-cell oncocytoma. Treatment and Prognosis. The treatment of choice is surgical excision, and the tumor does not tend to recur. Malignant transformation is uncommon, but malignant oncocytoma is now a well-established entity. Johns and associates reviewed the literature on malignant oncocytomas and reported three additional cases. Other well-documented cases have been those of Lee and Roth, and Gray and his coworkers.
Canalicular Adenoma
Histologic Features
The canalicular adenoma has a strikingly characteristic picture. It is composed of long columns or cords of cuboidal or columnar cells in a single layer. These single layers of cells are parallel, forming long canals. Some-times rows of cells are closely approximated and appear as a double row of cells showing a ‘party wall’. In some instances, cystic spaces of varying sizes are enclosed by these cords. The cystic spaces are usually filled with an eosinophilic coagulum. The supporting stroma is loose and fibrillar with delicate vascularity (Fig. 3-15). The tumor has, at times, been mistaken for an adenoid cystic carcinoma and care should be taken to prevent this error (Fig. 3-16). As Mader and Nelson have pointed out, the adenoid cystic carcinoma rarely occurs in the upper lip and is seldom freely movable. Occasionally the tumor may be multifocal and foci of tumor cells are found outside the main lesion.
Sebaceous Adenoma
Sebaceous adenoma is a rare benign tumor that accounts for 0.1% of all salivary gland neoplasms and slightly less than 0.5% of all salivary adenomas. The mean age at initial clinical presentation was 58 years (range 22–90 years). This tumor is more common in men. In a series of cases reported by Ellis et al (1991), 12 tumors were located in the parotid gland, 4 in the buccal mucosa, two in the submandibular gland, and three in the area of lower molars or retromolar region. The tumors ranged in size from 0.42–3.0 cm in diameter.
Conservative excision seems to be the treatment of choice. No recurrences have been reported.
Ductal Papilloma
Intraductal Papilloma
Microscopically, it exhibits a unicystic dilated structure. The cyst wall is lined by a single or double row of cuboidal and columnar cells, which extend into the cyst lumen as papillary projections having thin fibrovascular cores.
Cystadenoma
Histologic Features
Epithelial proliferation (Fig. 3-17) results in various sized cystic structures. The lining of these cystic structures varies from flattened to tall columnar cells, and cuboidal, mucous and oncocytic cells may also be seen. The lining thickness varies from one to three epithelial cells. Limited papillary growth with central connective tissue core is seen. Eosinophilic or slightly hematoxyphilic secretions are seen in the stroma. Dense fibrous connective tissue stroma with scattered inflammatory cells is present.
Malignant Tumors of the Salivary Glands
Acinic Cell Carcinoma: (Acinar cell or serous cell adenoma, adenocarcinoma)
Most salivary gland tumors arise from the epithelium of the duct apparatus, but occasionally lesions seem to show acinar cell differentiation. Acinic cell carcinoma is a malignant epithelial neoplasm in which the neoplastic cells express acinar differentiation. Some authors advocate the existence of both benign and malignant acinic cell neoplasms, whereas others are of the opinion that all acinic cell neoplasms are malignant. Unfortunately, the criteria for distinguishing between benign and malignant acinar cell tumors, if such a distinction exists, have not been clearly established. In an extensive study of acinic cell tumors of the major salivary glands by Abrams and his coworkers, it was concluded that most investigators believe that all tumors of this type have at least a low-grade malignant potential. By conventional use, the term acinic cell carcinoma is defined by cytologic differentiation towards serous acinar cells (as opposed to mucous acinar cells), whose characteristic feature is cytoplasmic PAS-positive zymogen-type secretory granules. In AFIP data of salivary gland neoplasms, acinic cell carcinoma is the third most common malignant salivary gland epithelial neoplasm after mucoepidermoid carcinoma and adenocarcinoma. In this data, acinic cell carcinoma comprised 17% of primary malignant salivary gland tumors or about 6% of all salivary gland neoplasms (cited by Ellis GL, Auclair PL, Gnepp DR, 1991).
Clinical Features
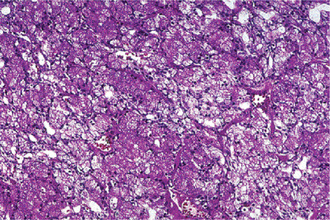
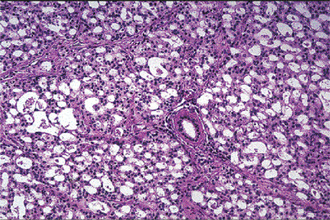
The acinic cell carcinoma closely resembles the pleomorphic adenoma in gross appearance, tending to be encapsulated and lobulated. Although this tumor has been reported occurring chiefly in the parotid, with more than 80% of the cases occurring in the parotid gland, it does occur occasionally in the other major glands and in the accessory intraoral glands (Fig. 3-18). The most common intraoral sites are the lips and buccal mucosa. The acinic cell carcinoma occurs predominantly in persons in middle age or somewhat older, the mean age being 44 years. It has also been encountered in 12% of the patients before the age of 20 years. Women were affected more than men (3 : 2). This tumor presents as a slowly growing, mobile or fixed mass of various durations. Usually asymptomatic but pain or tenderness is seen in over one third of the patients. Facial muscle weakness may be seen. Patients with bilateral synchronous tumors have been reported.
Histologic Features
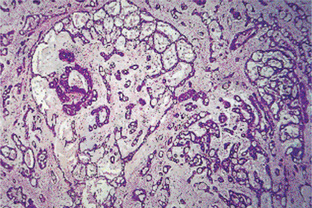
Connective tissue stroma is delicately fibro vascular collagenous tissue. Lymphoid elements are commonly found in parotid acinic cell carcinomas, a feature which is helpful in the diagnosis. Such features are not found in the intraoral tumors. Apparently the acinic cell carcinoma can arise from embryologically entrapped salivary gland tissue in lymph nodes in or near the parotid compartment. Although ‘clear cells,’ have been described in acinic cell carcinomas, they most likely represent cells altered by fixation or they may actually represent the component cells of a clear cell carcinoma (q.v.), a recently recognized entity (Figs. 3-19, 3-20).
Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma is a malignant epithelial tumor, first studied and described as a separate entity by Stewart, Foote and Becker in 1945. As the name implies, the tumor is composed of both mucus-secreting cells and epidermoid-type cells in varying proportions. Columnar and clear cells are also seen, and often demonstrate prominent cystic growth. It is the most common malignant neoplasm observed in the major and minor salivary glands. Mucoepidermoid carcinoma represents 29–34% of malignant tumors originating in both major and minor salivary glands. This carcinoma of the salivary glands accounts for 5% of all salivary gland tumors. The parotid gland is the most common site of occurrence. Intraorally, mucoepidermoid carcinoma shows a strong predilection for the palate.
Clinical Features
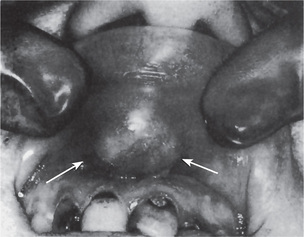
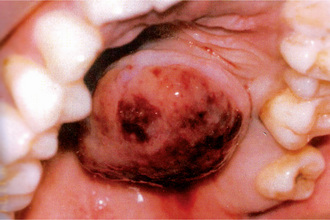
The tumor of low-grade malignancy usually appears as a slowly enlarging, painless mass which simulates the pleomorphic adenoma. Unlike the pleomorphic adenoma; however, the low-grade mucoepidermoid carcinoma seldom exceeds 5 cm in diameter, is not completely encapsulated and often contains cysts which may be filled with a viscid, mucoid material. In addition to palate intraoral tumors occur on the buccal mucosa, tongue and retromolar areas. Because of their tendency to develop cystic areas, these intraoral lesions may bear close clinical resemblance to the mucous retention phenomenon or mucocele, especially those in the retromolar area (Fig. 3-21).
Histologic Features
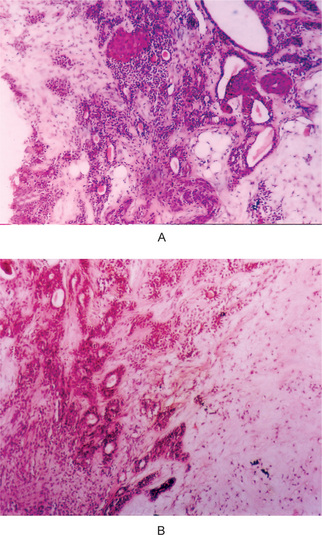
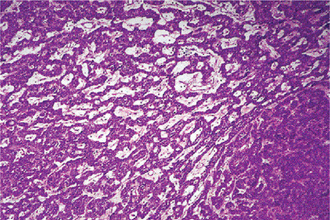
The mucoepidermoid carcinoma is composed of mucous secreting cells, epidermoid type (squamous) cells and intermediate cells. The mucous cells are of various shapes and have abundant, pale, foamy cytoplasm that stains positively for mucin stains. The epidermoid cells have squamoid features, demonstrate a polygonal shape, intercellular bridges and rarely keratinization. A population of cells that is often more important in recognizing mucoepidermoid carcinoma is a group of highly prolific, basaloid cells referred to as the intermediate cells. These cells are larger than basal cells and smaller than the squamous cells and are believed to be the progenitor of epidermoid and mucous cells. Occasionally clusters of clear cells can be present. These clear cells are generally mucin and glycogen free. Epidermoid cells, together with intermediate and mucous cells line cystic spaces or form solid masses or cords. Epidermoid and mucous cells may be arranged in a glandular pattern. The cysts may rupture liberating mucus which may pool in the connective tissue and evoke an inflammatory reaction (Fig. 3-22).
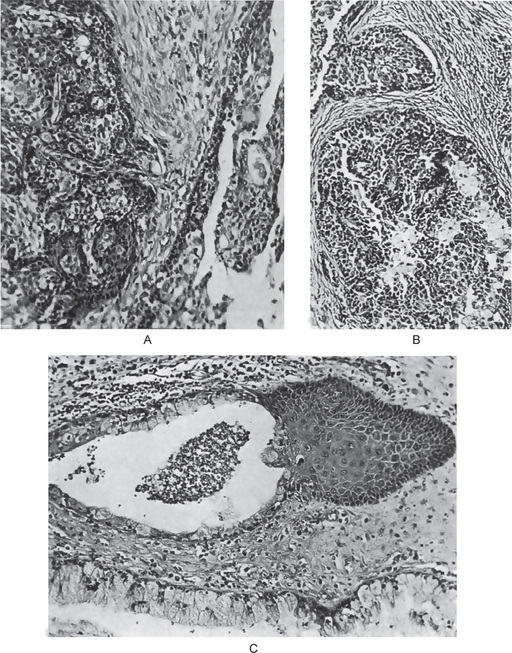
Figure 3-22 Mucoepidermoid carcinoma.
(A, B) Photomicrographs illustrate the association of the pale-staining, mucus-containing cells associated with darker staining epidermoid cells in a moderately high-grade mucoepidermoid carcinoma. (C) In one duct like structure the lining consists partially of squamous epithelium and partially of mucous cells From F Vellios: Am J Clin Pathol, 25: 147, 1955.
Mucoepidermoid carcinomas are graded as low-grade, intermediate-grade, and high-grade.
Low-grade tumors show well formed glandular structures and prominent mucin filled cystic spaces, minimal cellular atypia and a high proportion of mucous cells (Fig. 3-23).
Variants of Tumor
Treatment and Prognosis
Conservative excision with preservation of the facial nerve, if possible, is recommended for low- and intermediate-grade mucoepidermoid carcinomas of the parotid gland. The affected submandibular gland should be removed entirely. Radical neck dissection is performed in patients with clinical evidence of cervical node metastasis and is considered in any patient with a T3 lesion. Treatment for the minor glands is also primarily surgical. Some investigators have recommended postoperative irradiation only for high-grade malignancies, including high-grade mucoepidermoid carcinomas of the parotid glands. Few studies have assessed the role of chemotherapy and showed that high-grade mucoepidermoid carcinoma may show sensitivity similar to that of squamous cell carcinoma. Low-grade lesions had a five-year cure rate of 92%, whereas the intermediate-grade and high-grade lesions had a 49% 5-year cure rate.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


 . Benign Tumors of the Salivary Glands
. Benign Tumors of the Salivary Glands . Malignant Tumors of the Salivary Glands
. Malignant Tumors of the Salivary Glands . Other Carcinomas
. Other Carcinomas