Chapter 3
Tooth development
Martyn T. Cobourne1 and Paul T. Sharpe2
1 Department of Orthodontics, Dental Institute, King’s College London
2 Department of Craniofacial Development and Stem Cell Biology, Dental Institute, King’s College London; Guy’s Hospital
Teeth are unique and unusual organs in many respects. In humans, they are nonessential, but in all other animal species they are absolutely required for survival. In addition, their preservation in the fossil record makes them indispensable for understanding mammalian and in particular human evolution. Most interestingly, however, is the fact that teeth do not function as independent organs but only together, as a multi-organ unit or dentition, which is characteristic for each particular species. The essential processes underlying early tooth formation have been understood for some years; however, more recently scientists have begun to elucidate how teeth are formed at the molecular level.
This chapter will describe the essential histological processes associated with development of the tooth and provide some background with regard to the molecular mechanisms that control these processes. In addition, anomalies associated with tooth number that can occur in the human dentition are described.
The histology of tooth development
The first morphological evidence of tooth development occurs at around six weeks in utero, with the formation of a localized thickening or primary epithelial band in the epithelium of the putative upper and lower jaws. This thickening forms a continuous horseshoe-shaped sheet of epithelium around the lateral margins of the developing oral cavity. During the next week of development, the free margin of this band gives rise to two processes, which proliferate and invaginate into the underlying mesenchyme (Figure 3.1).
- The outer process or vestibular lamina is destined to degenerate and form the vestibule that demarcates the cheeks and lips from the tooth-bearing regions.
- The inner process or dental lamina will form the tooth buds.
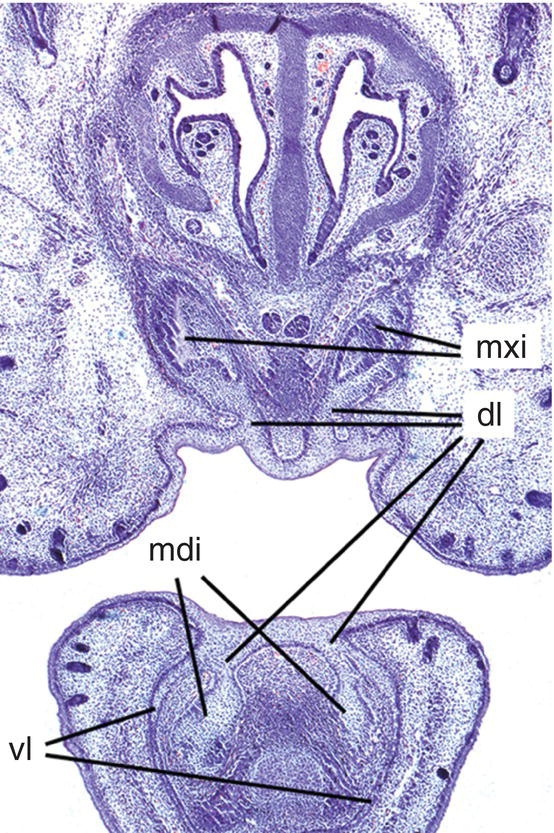
Figure 3.1 Early tooth development in the mouse incisor region. The maxillary and mandibular incisor tooth buds have formed (mxi and mdi, respectively) and are connected to the oral epithelium by the dental lamina (dl). In the lower jaw, the vestibular lamina (vl) is also clearly visible lateral to the tooth bud (hematoxylin and eosin).
Development of the coronal tissues
Rapid proliferation of discrete regions within the dental lamina produces a series of bud-like invaginations into the mesenchyme of the early jaws, with localized condensations of neural crest-derived ectomesenchymal cells appearing around the tips of these buds (Figure 3.2). During the late bud stage, the epithelium begins the process of folding, which is marked by formation of the primary enamel knot, a group of epithelial cells initially situated at the tip of the bud that act as a discrete, nonproliferating and transient signaling center, intimately involved with the regulation of tooth shape. Folding results in the formation of a cap-shaped enamel organ ultimately sitting over the dental papilla, which is a condensation of ectomesenchymal cells situated directly below the enamel organ. Further condensation of the papilla produces the dental follicle, which begins to encapsulate the epithelial cap-shaped enamel organ. Together, the enamel organ, dental papilla, and dental follicle constitute the tooth germ and will give rise to the essential structures of the tooth: the enamel, dentin-pulp complex, and periodontium, respectively. In multi-cusped teeth, such as premolars and molars, following the disappearance of the primary enamel knot, new secondary enamel knots form at the sites of the future cusp tips. These secondary enamel knots mark the first signs of a species-specific cusp pattern and herald the bell stage of tooth development. During this stage, the tooth germ undergoes a period of differential proliferation and histodifferentiation, whereby cells begin to differentiate into discrete regional structures (Figures 3.2 and 3.3). At the same time, the dental lamina begins to break down, separating the developing tooth from the oral epithelium.
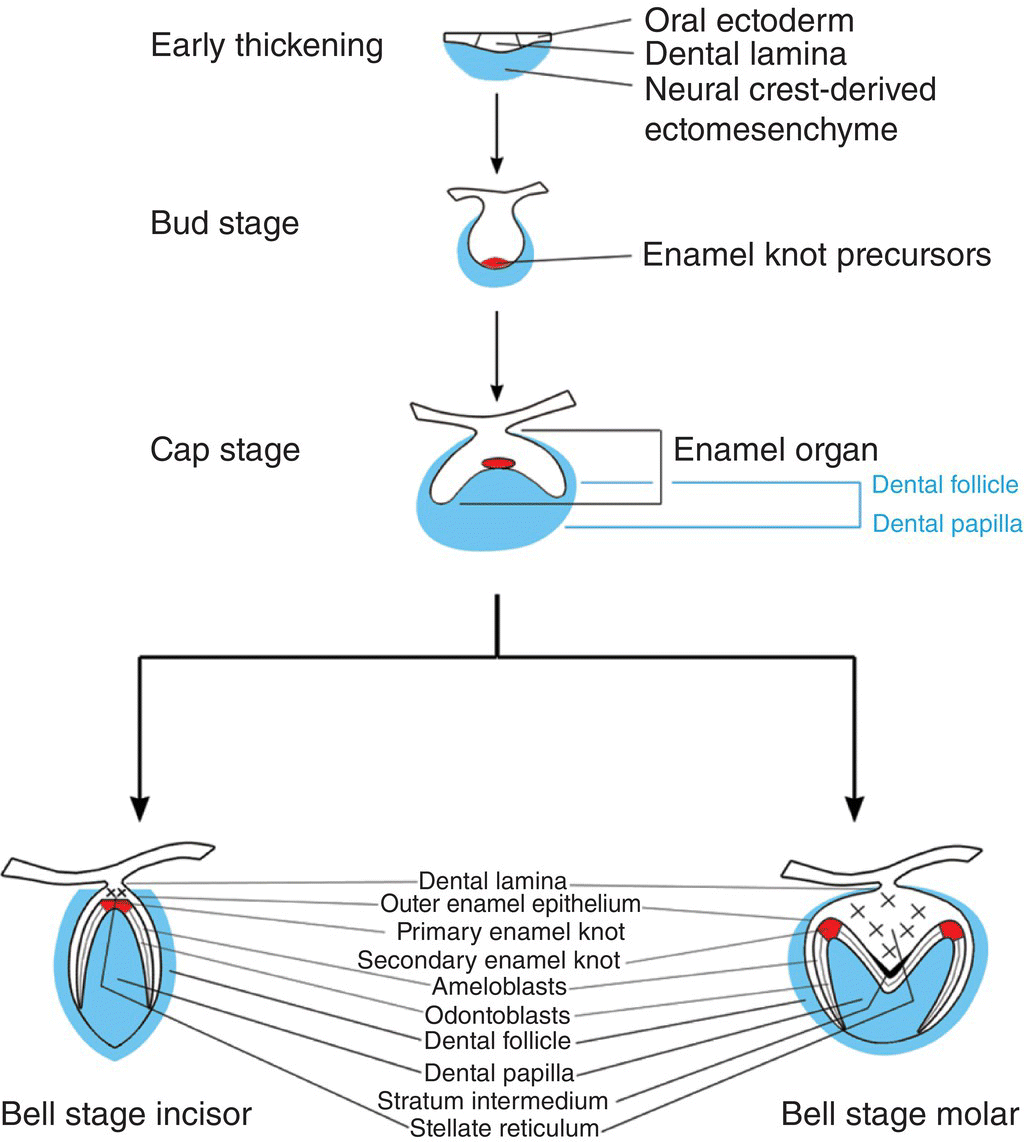
Figure 3.2 Schematic representation of mammalian tooth development. An early thickening of the oral epithelium gives rise to the tooth bud, which, following signaling mediated by the enamel knot, subsequently folds to form a cap stage tooth germ. The tooth germ consists of (1) the enamel organ, which is derived from ectoderm and will form the enamel of the tooth crown and (2) the dental papilla and follicle, which are derived from neural crest cells and will form the remainder of the tooth, including the dentin, root, and periodontal tissues. At the late cap stage, further folding of the enamel organ establishes future cusp shape as the tooth enters the bell stage. During the bell stage, specific populations of cells differentiate prior to hard tissue formation.
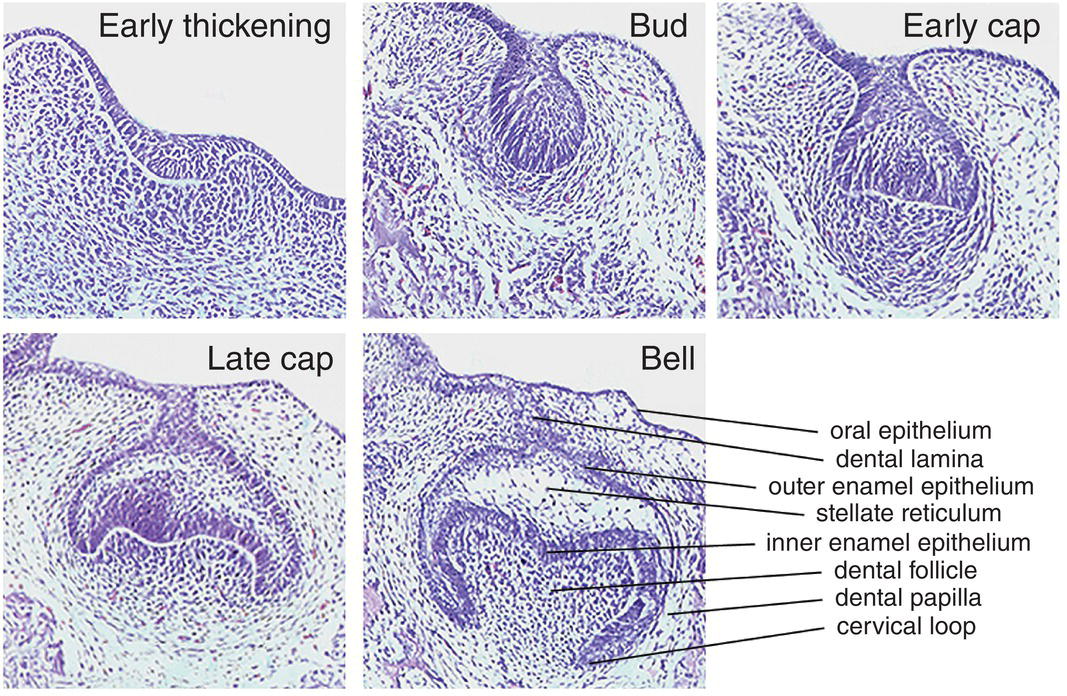
Figure 3.3 The essential histology of early tooth development. The first morphological sign occurs with the appearance of a localized thickening or early dental lamina within the oral epithelium. Rapid proliferation of this region results in formation of the tooth bud, with localized condensation of neural-crest-derived ectomesenchymal cells forming the dental papilla. Folding of the early bud results in first the cap and then bell stages, with progressive histodifferentiation within the bell ultimately producing the various cell populations that are required to generate the specific hard tissues that are found in the mature tooth. These sections show molar tooth development in the mouse, taking place between embryonic days 12 (early thickening) through to 15.5 (bell stage) (hematoxylin and eosin).
At the late bell stage, cells of the innermost layer of the enamel organ, the inner enamel epithelium, stop dividing and elongate. These changes preempt a reversal of polarity in these cells, and they very rapidly induce adjacent neural crest-derived cells of the dental papilla to differentiate into odontoblasts. Odontoblasts first appear at the sites of the future cusp tips and are responsible for secretion and mineralization of the dentin matrix as they differentiate along the length of the dental papilla adjacent to the internal enamel epithelium. Dentin formation is preceded by the formation of predentin, with this first layer acting as a signal to the overlying inner enamel epithelial cells to differentiate into ameloblasts and begin secreting the enamel matrix. The odontoblasts migrate in an apical direction and lay down coronal dentin, while ameloblasts migrate in a coronal direction and deposit enamel of the tooth crown (Figure 3.4).
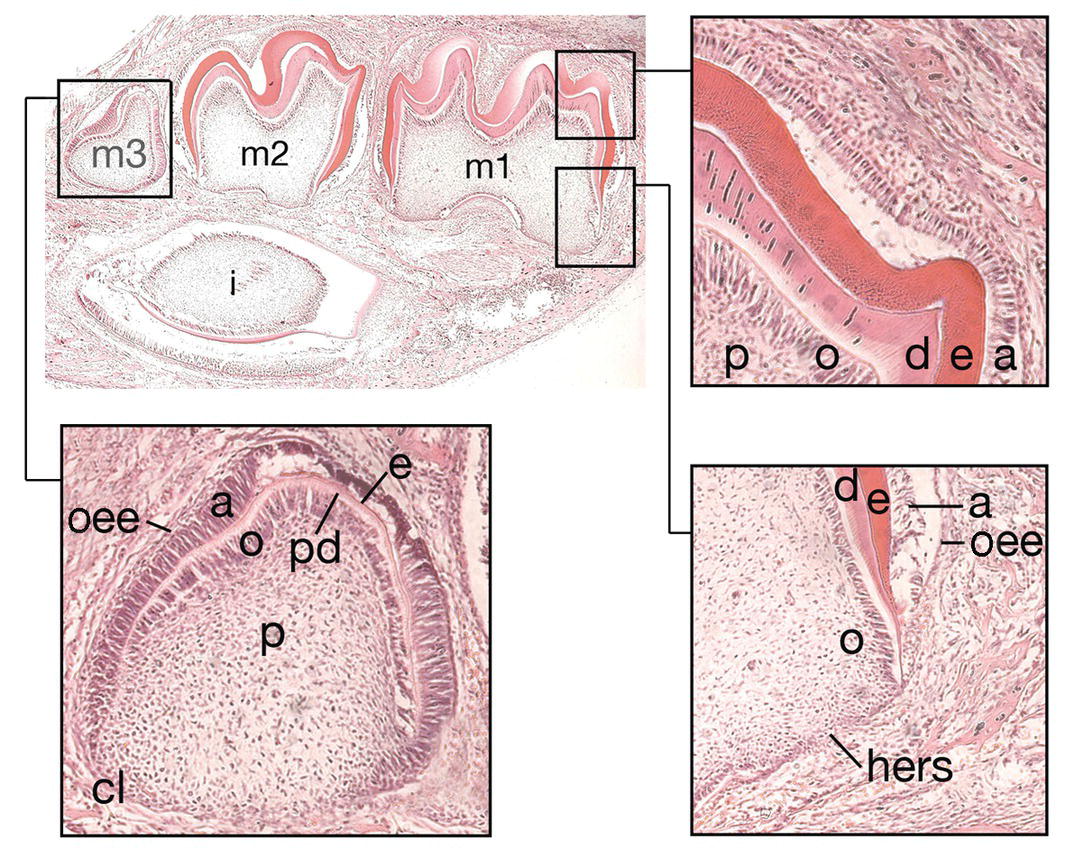
Figure 3.4 Hard tissue formation in the crown and root. During the late bell stage, cells within the inner enamel epithelium of the enamel organ differentiate into preameloblasts (a). This process, in turn, results in the differentiation of odontoblasts (o) within adjacent cells of the dental papilla (p) and the formation of, first, predentin (pd) and then dentin (d). The preameloblasts then become secretory ameloblasts and secrete enamel matrix (e). At the cervical loop (c), cells of the inner and outer enamel epithelium (oee) grow downwards as Hertwig’s epithelial root sheath (hers) to map out the future root. At 10 days of postnatal life in the mouse, the incisor (i) and molar dentitions (m1-3) are at different stages of development. The first molar (m1) is at the early stage of root development, while the second molar has just completed crown development. In the third molar (m3), early hard tissue formation is occurring within the crown (hematoxylin and eosin).
While these changes are taking place in the inner enamel epithelium, other regions of cellular differentiation also become apparent within the enamel organ. Around the periphery and confluent with the inner enamel epithelium at the cervical loop are the cuboidal cells of the outer enamel epithelium, which are thought to provide support to the tooth germ during hard tissue formation. Within the enamel organ, the star-shaped cells of the stellate reticulum are also thought to provide support and turgor to the enamel organ during amelogenesis. The maintenance of cell-cell contacts and presence of large fluid-filled spaces between these cells would seem to play a role in maintenance of coronal shape. In addition, situated adjacent to the inner enamel epithelium and stellate reticulum are the flattened cells of the stratum intermedium, which are thought to facilitate amelogenesis, although their precise function is unknown.
Development of the root
At the margins of the enamel organ the cells of the inner enamel epithelium are confluent with the outer enamel epithelium at the cervical loop. Growth of these cells in an apical direction between the dental papilla internally and the dental follicle externally forms a skirt-like sheet of cells known as Hertwig’s epithelial root sheath, which maps out the future root morphology of the developing tooth and induces the further differentiation of odontoblasts within adjacent regions of the dental papilla. In single-rooted teeth, the sheath forms a simple skirt; however, in multi-rooted teeth such as the molars, the sheath contains horizontal extensions which partition off regions within the dental papilla and map out the individual roots. Rapid degeneration of the root sheath leads to the exposure of cells within the dental follicle to newly formed root dentin and differentiation of these mesenchymal cells into cementoblasts, which begin to deposit cementum onto the root surface. Surrounding the enamel organ, the outer cells of the dental follicle begin to produce the alveolar bone and collagen fibers of the periodontium, which ultimately will anchor the tooth into its bony socket within the jaw. The developing tooth remains housed in this cavity of alveolar bone until the process of eruption begins (Figure 3.4).
Successional and accessional tooth development
Humans have a primary, or deciduous, dentition, which begins eruption at around 6 months of age and is usually complete by 2-1/2 years of age. This is replaced by the secondary, or permanent, dentition, which begins with eruption of the first molars at 6 years of age and is not complete until the third molars erupt (if they are present) during the late teenage years. Within the secondary dentition there are two types of teeth, successional and accessional.
- Successional teeth have primary predecessors and consist of the incisors, canines, and premolars.
- Accessional teeth have no primary precursors and consist of the three secondary molar teeth.
During development of the secondary dentition, successional teeth form on the lingual (or palatal) side of the primary tooth germ as a result of localized proliferation within the dental lamina associated with each primary tooth germ. The accessional teeth form from a backward extension of the original dental lamina (Figure 3.5).
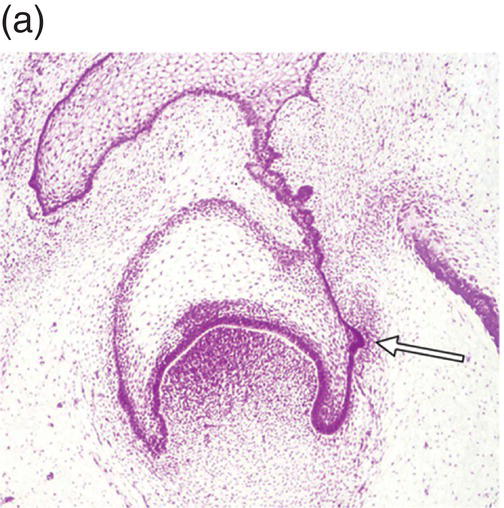
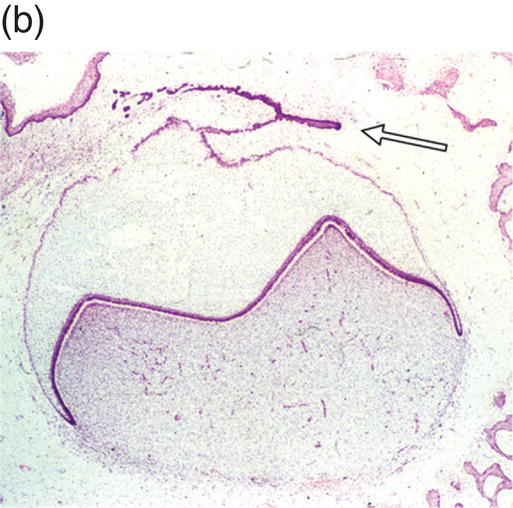
Figure 3.5 Successional teeth form via localized proliferation within the dental lamina associated with each primary tooth germ (a, arrow). Accessional teeth form as a posterior extension of the dental lamina (b, arrow) (hematoxylin and eosin).
(Courtesy of Dr. Barry Berkovitz.)
Chronology of human tooth development
Tooth development begins in the human embryo at around 6 weeks of development, with the appearance of the primary epithelial band within the early jaw primordia. This is followed a week later by the initiation of odontogenesis and by 11 weeks, the primary tooth germs have reached the early cap stage (Figure 3.6). The bell stage is reached at around 14 weeks of intrauterine development, with hard tissue formation beginning at around week 16. At this stage, the first secondary tooth germs begin to appear, with the successional incisors and accessional first molars beginning their development. At birth, calcification of the primary dentition is well underway, with coronal development complete in all these teeth by the age of 1. In addition, the first evidence of secondary tooth calcification (associated with the first molars) is usually seen at birth. During the first few years of life, the primary dentition erupts, with root formation being complete by the age of 3. Thus, from this age the primary dentition is regarded as complete.
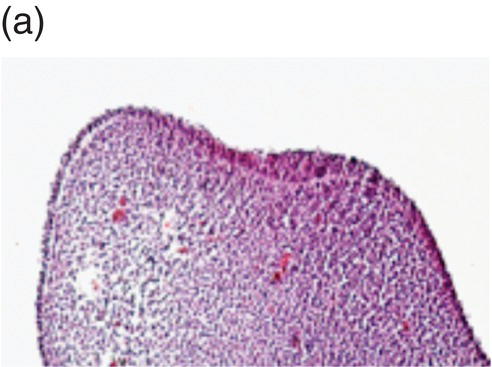
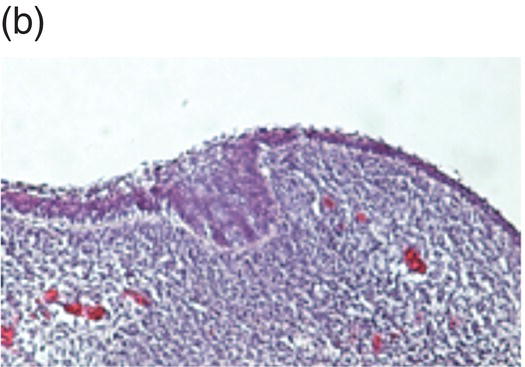
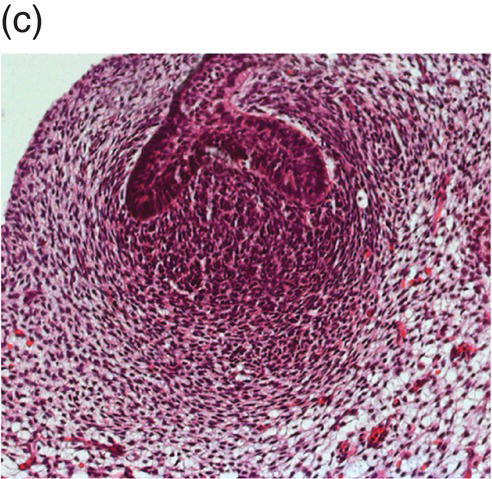
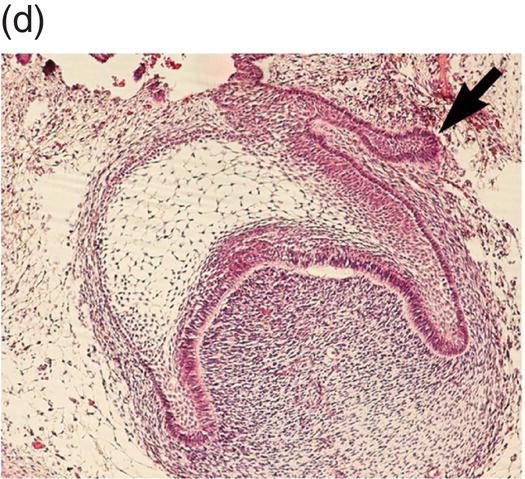
Figure 3.6 Histology of human tooth development. (a) Early thickening; (b) Bud stage; (c) Cap stage; (d) Bell stage (note early development of the successional tooth germ, arrow) (hematoxylin and eosin).
(Courtesy of Dr. Ana Angelova.)
The first molars of the secondary dentition normally have completed their coronal development by 3 years of age and will erupt around the age of 6 years. Their root development is not complete until around 10 years of age, and during this time the secondary incisor dentition will have erupted and replaced the primary teeth. From the age of 10 to 12 years, the canine, premolar, and then second molar teeth will erupt, with root formation completed at around 16 years. Finally, the third molars, which began their development during postnatal life and only began their coronal calcification at around 10 years of age, will erupt during the late teenage years, although these teeth are often absent or simply fail to erupt at all. All root development in the secondary dentition is usually complete by the mid 20s (Figure 3.7).
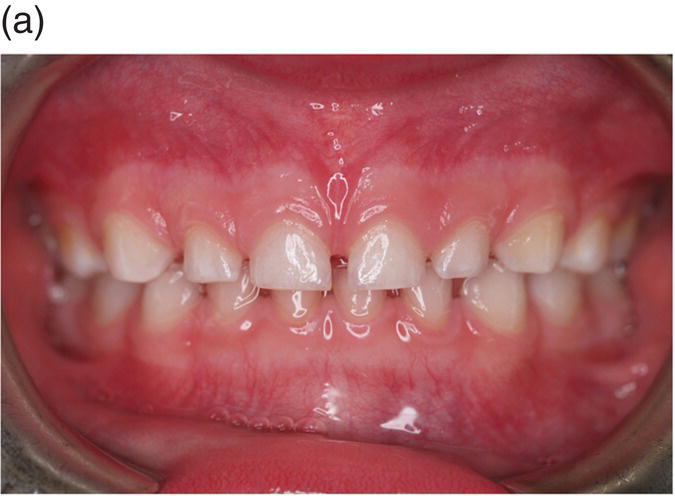
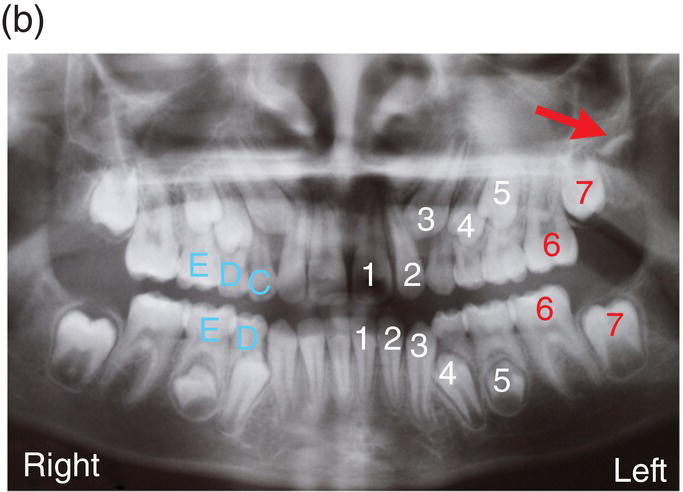
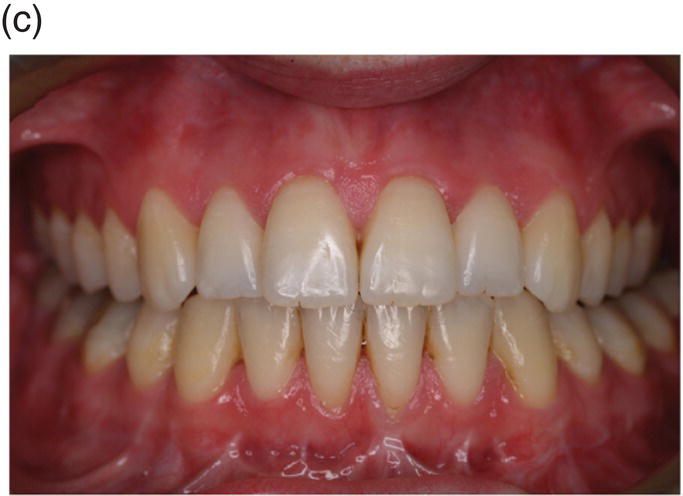
Figure 3.7 The primary (deciduous), mixed and secondary (permanent) human dentitions. (a) The primary dentition of a 4-year-old boy; (b) Panoramic radiograph of the mixed dentition in a 10-year-old girl. Arch development is symmetrical, with the exception of a solitary, early developing third molar in the upper left quadrant (red arrow). On the left, successional and accessional teeth of the secondary dentition are identified in white and red, respectively. Primary teeth are identified on the right in pale blue (note that all the premolars [4, 5], the upper permanent canines [3] and all second permanent molars [7] are unerupted and that the lower primary canines [C] have been exfoliated; (c) Permanent dentition in a 30-year-old woman.
The biology of early tooth development
The development of the mammalian dentition can be considered to involve a sequence of closely related events.
- Initiation is the process that determines those sites along the oral axis where teeth are destined to form.
- Patterning of the dentition is responsible for determining the type of tooth, for example incisor or molar, that will form within a specific region.
- Morphogenesis ultimately generates a tooth with characteristic shape.
Initiation essentially links patterning with morphogenesis, but integral to all three of these mechanisms is differentiation, whereby the constituent epithelial and mesenchymal cells of the tooth germ ultimately form the specific structures of the adult tooth.
Embryonic origins of the dental tissues
The basic histology of mammalian tooth development demonstrates that this process derives from two principal cell types within the early jaws, epithelium and neural crest-derived ectomesenchyme. The epithelium is ultimately derived from the epiblast of the early embryo, and mammalian fate mapping studies have suggested that this component of the developing tooth is derived from ectoderm; however, in other species such as fish and reptiles teeth can develop within the pharynx from endoderm.
Mesenchyme of the early jaws is predominantly composed of cells derived from the cranial neural crest. The vertebrate neural crest is a pluripotent cell population, derived from the lateral ridges of the neural plate during early embryogenesis. Neural crest cells disperse from the dorsal surface of the neural tube and migrate extensively throughout the embryo, giving rise to a wide variety of differentiated cell types. Cranial neural crest-derived ectomesenchyme contributes to the formation of condensed dental mesenchyme at the initial bud stage and subsequently to formation of the dental papilla and follicle in the developing tooth germ. Genetic fate mapping studies have demonstrated a cranial neural crest origin for odontoblasts, dentin matrix, pulp tissue, cementum, and the periodontal ligament of mature teeth (Figure 3.8).
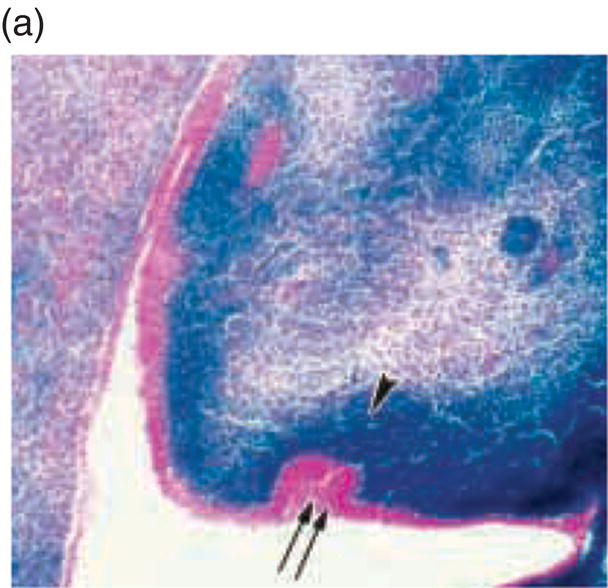
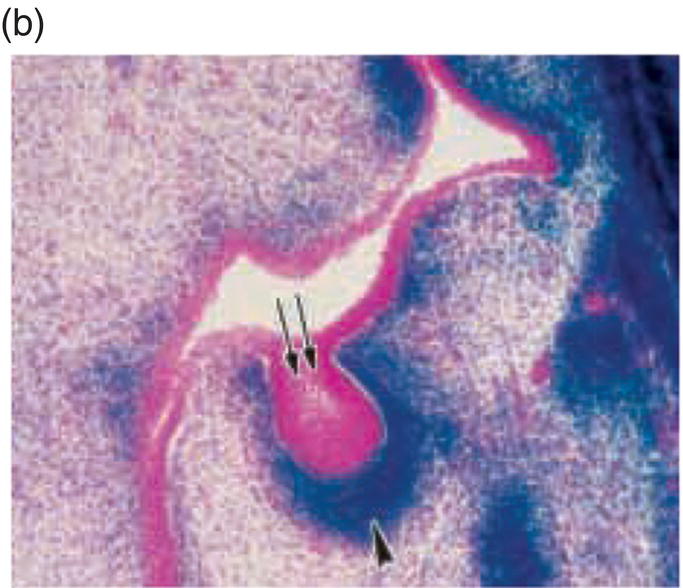
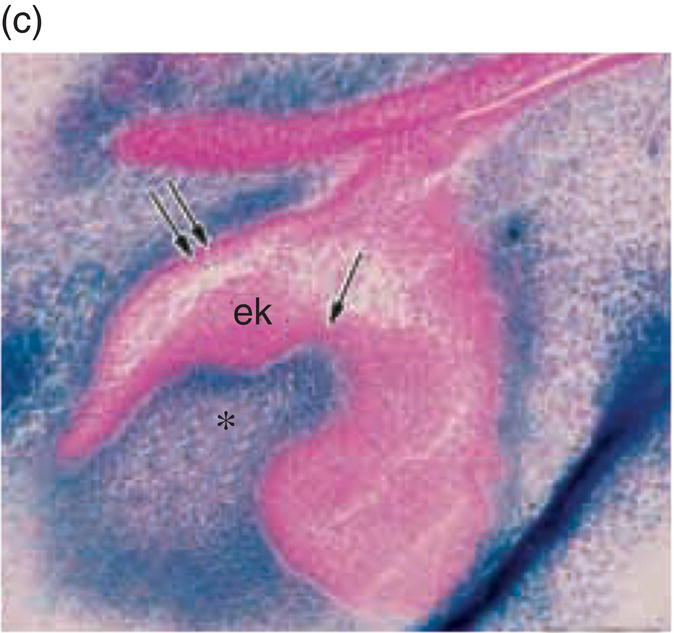
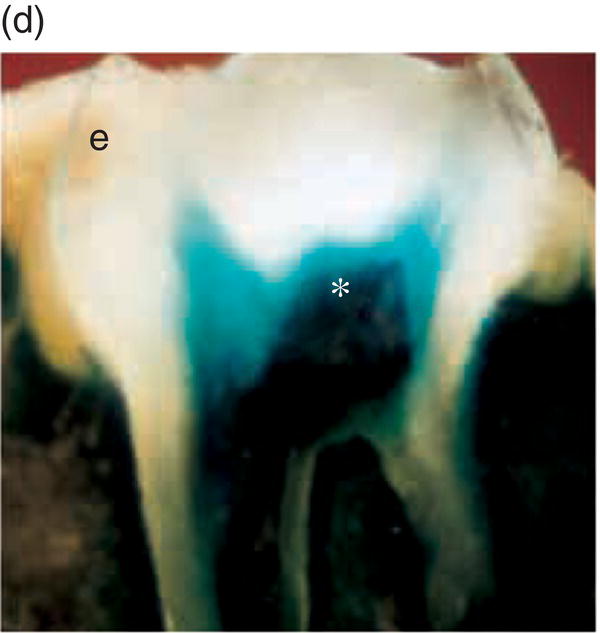
Figure 3.8 A cranial neural crest origin for odontoblasts, dentin matrix, pulp tissue, cementum and periodontal ligament. Recently, Chai and colleagues have used the expression of a Wnt1 reporter gene as a genetic marker to follow neural crest migration and differentiation in the mouse. All cranial neural crest cells destined for the early jaws are ultimately derived from Wnt1-expressing cells in the central nervous system. These investigators generated mice exhibiting ubiquitous expression of a lacZ reporter in neural crest precursors (blue color), which allowed staining and identification of these cells at all stages of tooth development. This experiment has definitively demonstrated a cranial neural crest cell contribution to the developing dental papilla and follicle. Arrows indicate the epithelial component of the tooth germ, while the arrowheads indicate neural crest-derived cells. (a) Dental lamina (arrows) and dental mesenchyme (arrowhead); (b) Early bud stage (arrows), dental mesenchyme (arrowhead) (c) Cap stage, inner enamel epithelium (single arrow), outer enamel epithelium (double arrow), enamel knot (ek), dental papilla (*); (d) Adult maxillary molar; enamel (e), dentin and pulp (*). [http://dev.biologists.org])
(Reproduced and adapted with permission from Y. Chai, X. Jiang, Y. Ito, et al. (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671–1679.
How the dentition is patterned
In the mammalian dentition the teeth form a series of homologous structures whose differences along the jaw axis can be described in terms of changes in shape and size. Based upon the analysis of adult dentition, two classic theories have been proposed to account for the developmental mechanisms responsible for patterning this axis (Figure 3.9).
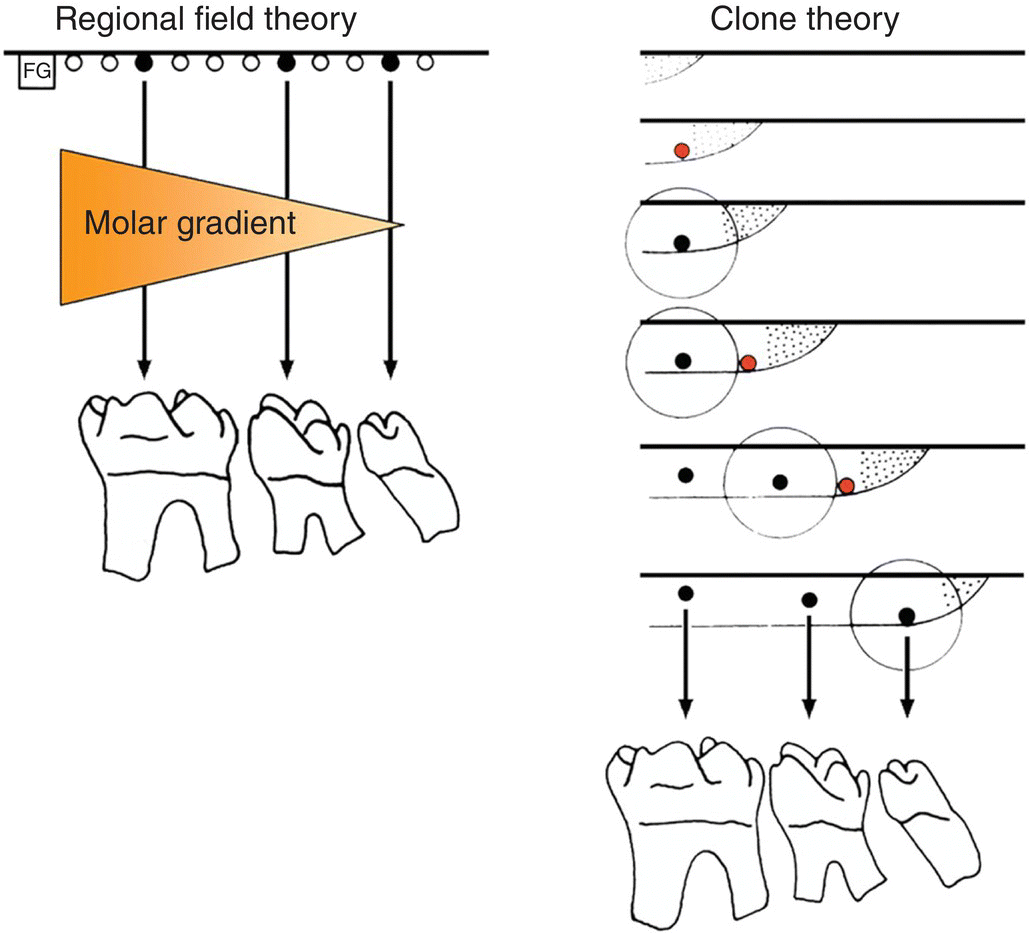
Figure 3.9 Regional field and clone theories of dental patterning. According to the regional field theory, identical tooth primordia (black dots) are acted upon by a morphogenetic substance (orange shading) generated by a field generator (FG). The substance has a graded concentration in the field, which results in each primordium (black dots) developing into a specific tooth (in this case, a molar) with a different morphology. According to the clone theory, the gradient of final tooth form is related to the times at which tooth primordia (black dots) are initiated. The stippled region represents the growing margin of the clone with an associated zone of inhibition (circle). The red dots represent tissues that have reached the critical stage to form a new primordium.
(Adapted from Lumsden, 1979.)
Butler (1967) proposed that a simple morphogenetic field determined tooth shape. In this regional field theory all the tooth primordia were proposed to be initially equivalent, the shapes that the teeth ultimately develop into being controlled by substances or signals generated elsewhere in the jaw primordia. These signals would have a graded concentration along the developing jaw axis, such that each tooth primordium developed differently according to its position relative to the source of the signal. However, within this theory the generation of the primary pattern of the dentition, or the initial primordia themselves, is not accounted for. In contrast, Osborne (1978) suggested a clone model, whereby all the teeth are suggested to develop from a single clone of mesenchymal cells. This mesenchymal clone grows to form the individual teeth in sequence. Thus, ectomesenchyme enters the jaw and, following interaction with the oral epithelium, a clone of cells is established for a specific tooth class. The clone grows forward or backward within the jaw, but surrounding each newly initiated tooth primordium is a region of inhibition, which prevents the formation of a second primordium. Once the growing clone has escaped this region of inhibition, a new primordium is initiated. Gradients of tooth shape are related to the sequence in which they are initiated, successive mitoses of the clone gradually altering the shape potential of newly initiated primordia. The fundamental principle of the clone theory, unlike the regional field theory, is that the shape of the tooth is determined from the moment its primordium has been initiated. The initiation of primordia is a prerequisite of both theories and this is a property of the oral epithelium. Isolated presumptive first molar tissue can give rise to all three molar teeth in their normal sequence when transplanted. These findings are consistent/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


