Reconstruction of the Auricle
Anatomy and Embryology
Topographic landmarks of the auricle are illustrated in Figure 22-1. The cartilaginous framework is composed of several distinct three-dimensional components. Medially, these components are the conchal, antihelical, antitragal, helical, and lobular complexes.1 These units are critical in planning the reconstruction of defects involving cartilage replacement.2
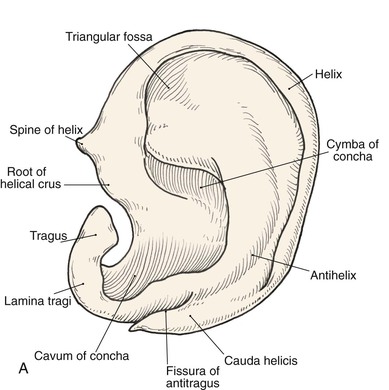
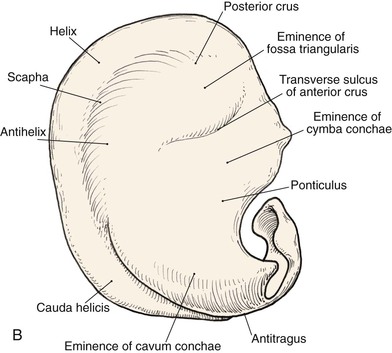
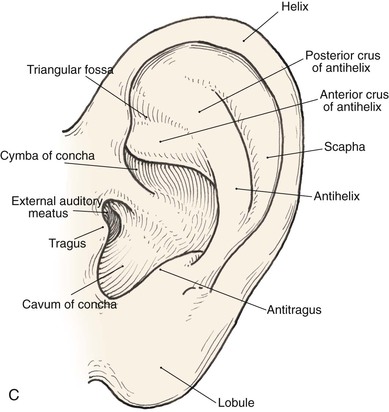
FIGURE 22-1 Auricular cartilage anatomy. A, Lateral aspect. B, Medial aspect. C, Topographic landmarks of the lateral surface of auricle.
The arterial supply to the auricle is derived from branches of the superficial temporal artery and the postauricular artery. Both vessels arise from the external carotid artery.3 The medial surface of the ear is supplied by the postauricular artery. The lateral surface is supplied by both the postauricular and superficial temporal arteries, creating two arterial networks.4 The triangular fossa—scapha network is supplied by the helical branch of the superficial temporal artery. The conchal network is supplied by the postauricular artery, which provides septal perforators to the conchal floor (Fig. 22-2). Flap planning and design should give due consideration to these arterial networks. Venous drainage is through the postauricular vein, which drains into the external jugular system. There is supplemental venous drainage from the superficial temporal and retromandibular veins. Lymphatic drainage of the auricle is primarily to the preauricular, infra-auricular, and mastoid lymph nodes.
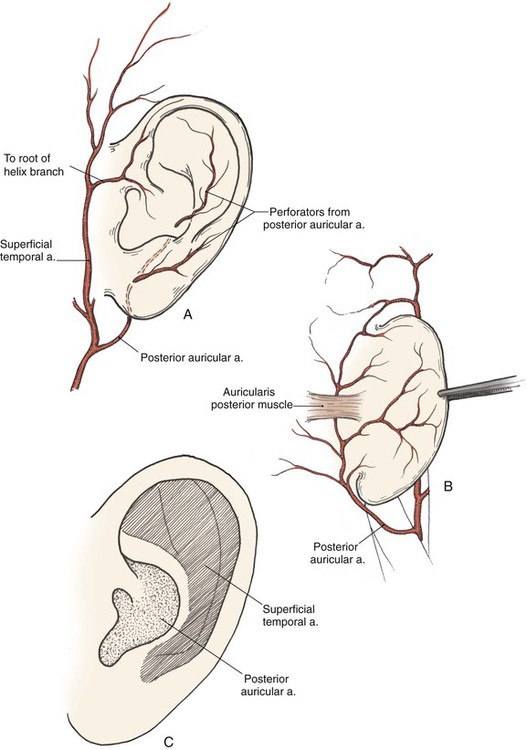
FIGURE 22-2 Arterial blood supply to auricle. A, Lateral aspect. B, Medial aspect. C, Lateral aspect of auricle, with indicated zones supplied by superficial temporal and postauricular vascular systems.
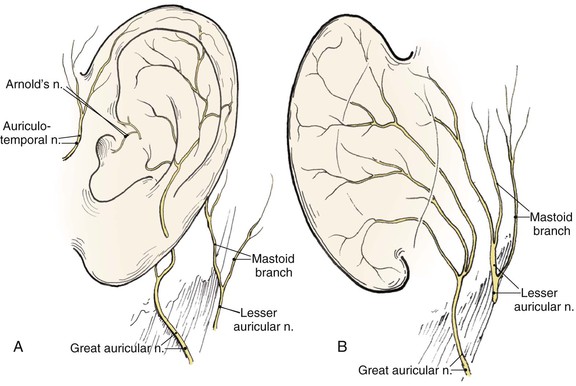
The auricle is innervated by contributions from both cranial and spinal nerves. They include the great auricular nerve (C2, C3), the auriculotemporal nerve (V3), the lesser occipital nerve (C2, C3), and a branch of the vagus nerve (X, Arnold’s nerve) (Fig. 22-3). The great auricular nerve divides into an anterior and posterior branch. The anterior branch supplies the lower half of the lateral aspect of the auricle. The posterior branch supplies a similar area on the medial surface of the auricle. The auriculotemporal nerve supplies the superolateral surface of the auricle. The lesser occipital nerve supplies the superior aspect of the ear on the medial side. The concha is supplied by Arnold’s nerve.
The auricle arises embryologically from the first and second branchial arches.5 Hillocks appear in these arches during the sixth week of gestation. The anterior hillock gives rise to the tragus, the root of the helix, and the superior helix; the posterior hillock becomes the antihelix, tragus, and lobule. The first branchial groove forms the external auditory meatus and concha.
Auricular Architecture
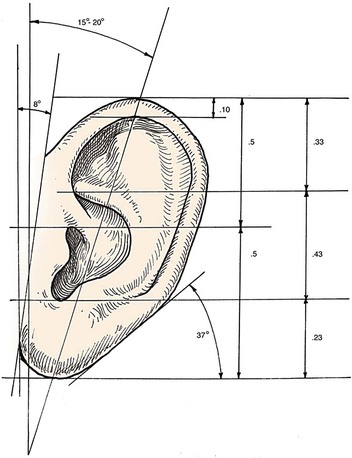
Analysis of the dimensions and proportions of the external ear is important for planning reconstructive procedures.6–9 The vertical height of the ear roughly matches the distance between the lateral orbital rim and the root of the helix, at the level of the eyebrow. Ear width is approximately 55% of its length (Fig. 22-4), and the helical rim protrudes 1- to 2.5-cm from the skull. The angle of protrusion from the skull typically ranges from 25° to 30°. The vertical axis of the ear is inclined posteriorly at an angle of approximately 15° to 20°.10,11 The original anatomic descriptions of auricular orientation suggested that the long axis of the ear was roughly parallel to the dorsum of the nose, but it is now widely recognized that the ear is rotated 10° anterior to that plane. The superior level of the ear matches the level of the lateral eyebrow. To account for individual variations in topographic features, positioning, and symmetry, the contralateral ear is used as a template in planning reconstruction, rather than strict adherence to textbook-defined norms. The most important principle of auricular reconstruction is that size, location, and orientation of the reconstructed ear be similar to the normal ear. The dominant features that make an ear recognizable are the helix, tragus, antitragus, and conchal bowl.8,9
General Principles
Auricular defects may be divided into defects of cutaneous coverage, with or without intact cartilage, and defects that are full thickness. Small cutaneous defects of the helical rim may be reconstructed with primary wound closure and occasionally require small excisions of cartilage to avoid distortion. Defects of the densely adherent lateral auricular skin can rarely be closed primarily, whereas those of the more pliable medial surface can often be repaired primarily. The pliability of medial auricular skin permits the harvest of relatively large postauricular skin grafts with primary closure of the donor site. Lateral cutaneous defects that have intact cartilage are best treated with skin grafts, provided there is intact perichondrium over the exposed cartilage. The contralateral postauricular skin is an excellent donor site for a full-thickness skin graft used to resurface the lateral ear. Preauricular skin may also be used with minimal donor site morbidity. In cases in which the perichondrium is absent, resulting in an avascular surface, cartilage may be removed if it is not critical to auricular shape (see examples in Chapter 15). Windows created through the cartilage provide surface contact between the graft and the vascularized subcutaneous tissue of the medial aspect of the auricle. The cartilage of the concha, fossa triangularis, and scapha are amenable to partial removal without loss of structural integrity of the ear. The cartilaginous windows provide access to the perichondrium of the medial aspect of the ear, which in turn provides sufficient vascularity for successful skin grafting.
Surgical approaches for reconstruction of defects of skin and underlying cartilage include converting the defect into a full-thickness wedge-shaped excision, followed by primary wound closure, and employing a composite graft or flap that incorporates structural support. The size and location of the defect dictate which alternative is most appropriate. Defects less than 1.5 cm of the helix or antihelix are best repaired by conversion to a wedge-shaped excision. The wound is then repaired primarily and results in a minimally visible scar. Whereas this approach does reduce vertical height of the ear, the difference with the contralateral ear is usually not conspicuous. When a wedge-shaped excision of the auricle is performed, excessive wound closure tension is avoided by removing a portion of the concha to accommodate advancement of the two borders of the ear defect. If this is not performed, cupping and distortion of the ear will occur. Medium-sized helical defects, from 1.5- to 2-cm, can be managed by composite grafting from the contralateral ear.12 Typically, a composite graft half the size of the defect is transplanted from the opposite ear, thereby creating two ears of equal size (Fig. 22-5). The cartilage edges of the graft and the recipient site are securely approximated with a row of interrupted sutures. Graft immobilization is essential for proper healing and can be accomplished by use of bolster sutures to secure a splint over the ear. De-epithelialization of the medial aspect of the composite graft and its implantation into a subcutaneous recipient site have been described. The graft is released from its soft tissue attachment in a second surgical stage. This approach is designed to increase graft survival. Transfer of a composite graft from the contralateral ear exposes both ears to some risk of infection and deformity, so its use must be carefully considered in view of this risk.

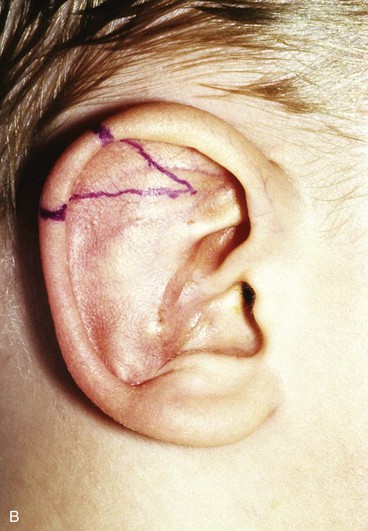
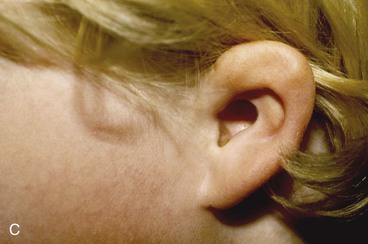
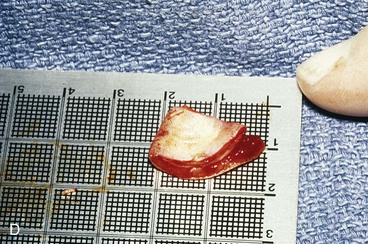
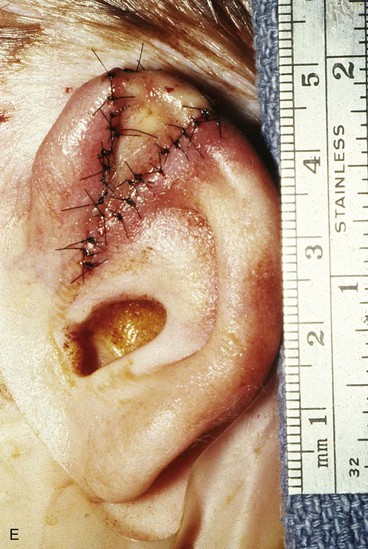
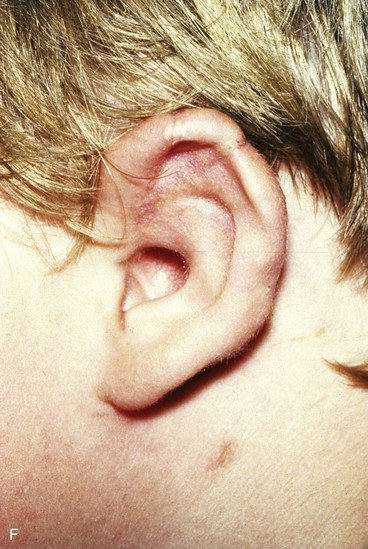
FIGURE 22-5 Left congenital auricular deformity with more than 20-mm difference in vertical height between auricles. A, Preoperative view. B, Donor ear composite graft outlined (half size of recipient deficit height). C, Preoperative view of affected ear. D, Harvested composite graft. E, Graft in place. Vertical height increased by 1 cm. Epithelium removed from medial aspect of graft, and medial aspect implanted in subcutaneous pocket. F, Postoperative result at 12 months.
Many local flaps have been described for repair of full-thickness auricular defects.13–22 Chondrocutaneous composite advancement flaps are best suited to repair of full-thickness helical rim defects. Helical chondrocutaneous composite flaps take advantage of the available tissue of the remaining helical rim and the redundant lax skin of the lobule. Anteriorly, a V-Y advancement of the helical root designed as an island flap is usually combined with a posterior chondrocutaneous composite advancement flap to achieve closure of helical rim defects (Fig. 22-6). Chondrocutaneous composite flaps may be advanced with the medial skin intact or incised. Although Antia originally described keeping the medial skin intact, there is greater ease of flap advancement with at least a limited incision of the medial skin. The arterial network of the helix-scapha region allows the creation of long chondrocutaneous composite flaps on a narrow pedicle. Depending on the size of the helical defect, Burow triangles are excised to facilitate advancement of the flaps (Figs. 22-7 and 22-8).23 Chondrocutaneous flaps in the form of transposition flaps may also be used for reconstruction of nonhelical defects.
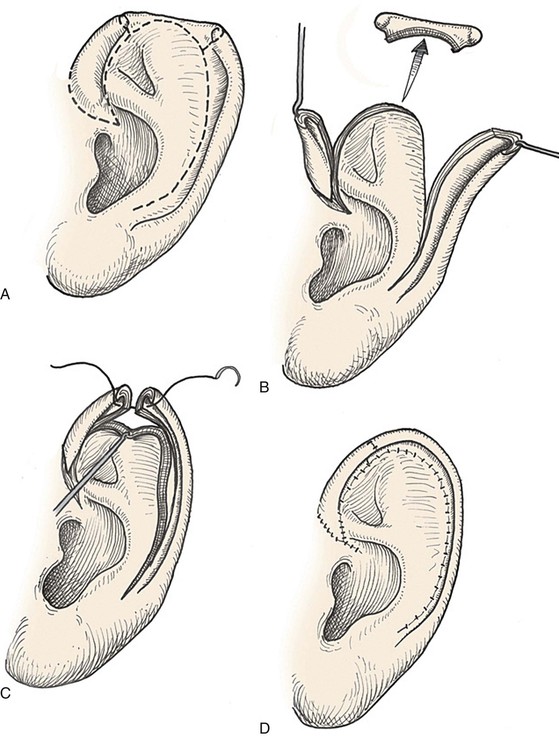
FIGURE 22-6 Auricular chondrocutaneous composite flaps. A, Helical defect. Broken lines indicate incisions through skin and cartilage. B, Island advancement flap consisting of helical root advanced on medial soft tissue pedicle. Remaining helix pedicled on earlobe. C, Flaps advanced. D, Wound closure.
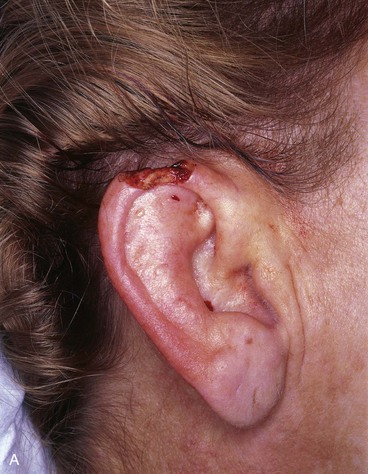
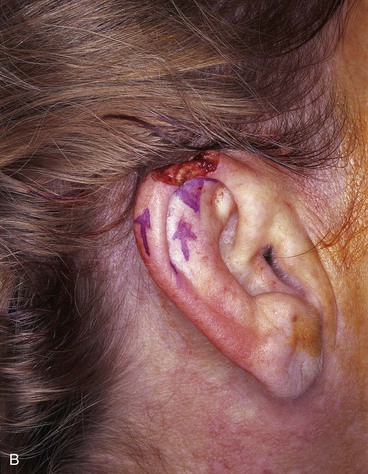
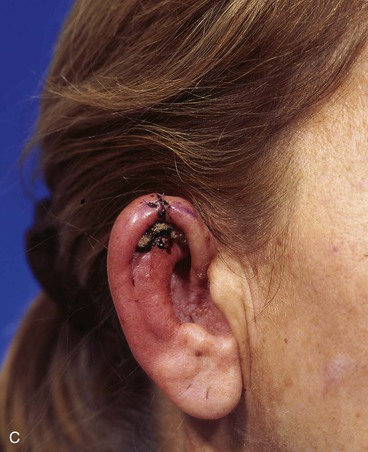
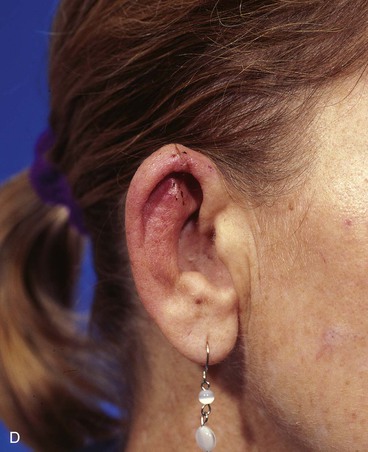
FIGURE 22-7 A, A 1.5-cm full-thickness defect of helix. B, Unilateral chondrocutaneous composite helical advancement flap planned for repair. Burow triangle marked for excision to facilitate advancement. C, Flap in place. Bolster dressing secured with sutures to maintain contour of scapha. D, Postoperative result at 7 days. (Courtesy of Shan R. Baker, MD.)
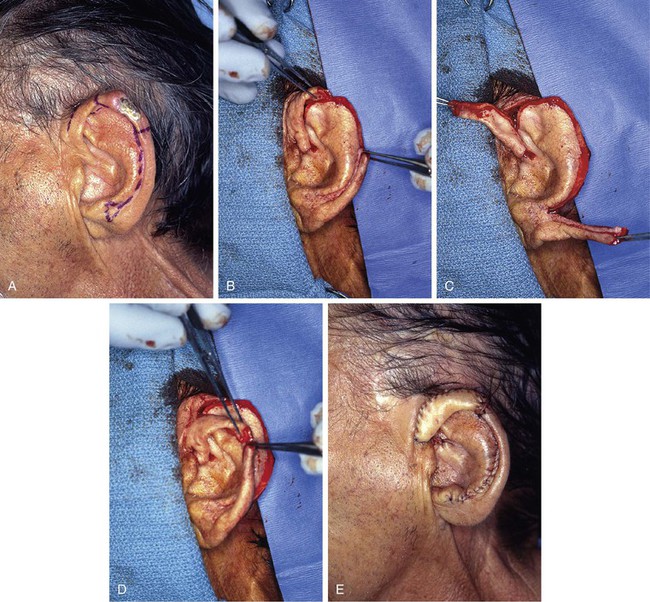
FIGURE 22-8 A, Squamous cell carcinoma of helix marked for excision. Broken lines outline planned incisions for bilateral chondrocutaneous composite helical advancement flaps after excision of tumor. B, Tumor excised. One-third of helix removed. C, Advancement flaps incised. D, Apposition of flaps demonstrates requirement for reduction of scapha and superior antihelix. E, Wound closed after reduction. (Courtesy of Shan R. Baker, MD.)
Both postauricular skin and preauricular skin serve as ideal donor sites for the creation of interpolated tube flaps. These flaps are used to reconstruct longer segments of the helical rim that are too large for reconstruction with a chondrocutaneous advancement flap. Initially, the flap is dissected as a bipedicled flap that is tubed on itself (Fig. 22-9A). After 3 weeks, one end of the cutaneous tube is detached from its origin and secured to one border of the helical rim defect. Three weeks later, the other end of the tube is incised and inset in the other border of the helical defect. Interpolated tube flaps are most useful for reconstruction of defects confined to the helical rim that are more than 2.5 cm in length (Fig. 22-10E, F).
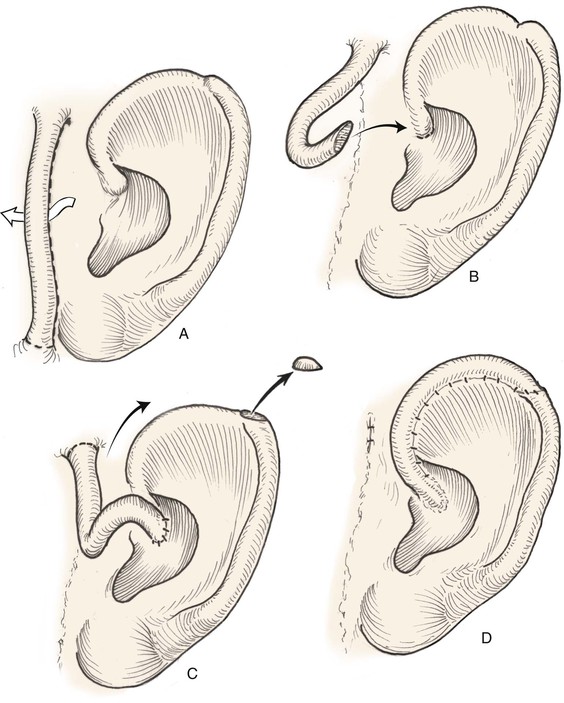
FIGURE 22-9 Preauricular interpolated cutaneous tube flap for repair of defects confined to helix. A, First stage: tube created from preauricular skin. B, Second stage: division of inferior pedicle and flap transfer to helical root. C, Third stage: division of superior pedicle and flap inset. D, Final appearance after inset.
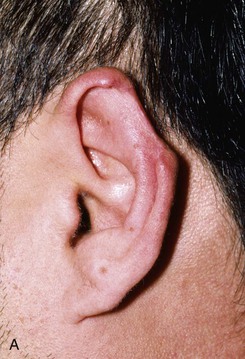
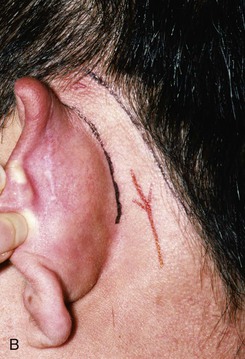
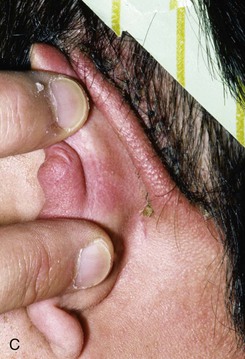
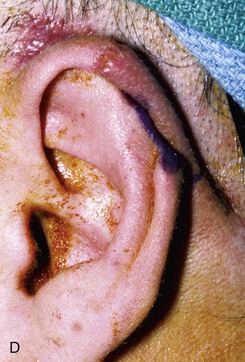
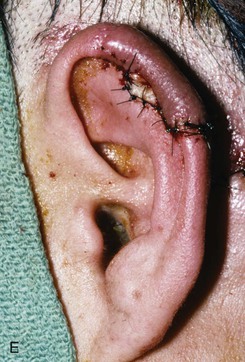
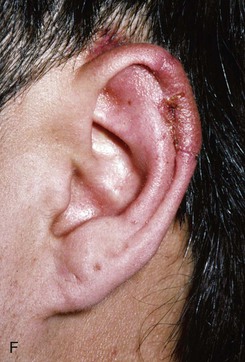
FIGURE 22-10 A, Helical defect. B, Postauricular interpolated cutaneous tube flap designed. C, Three weeks after first-stage tube creation. D, After second-stage transfer of flap to ear. E, Third-stage flap inset. F, One month after third surgical stage.
Postauricular island advancement flaps work well for conchal reconstruction and repair of full-thickness defects of the nonhelical portions of the ear.24,25 The postauricular flap is based on the auricular branch of the postauricular artery. The flap is designed as an island based on a subcutaneous tissue pedicle. A significant advantage is that it provides a single-stage repair with easy closure of the postauricular donor site. When it is designed as an island, the flap is outlined and incised deeply through the postauricular muscle. Posteriorly, the mastoid periosteum is incised, and the dissection proceeds in a superior and anterior direction. Care is taken to preserve the vascular supply to the flap as it enters the flap inferiorly. With the subcutaneous pedicle intact, the flap is advanced into its respective conchal or nonmarginal auricular defect (Fig. 22-11). Postauricular flaps may also be developed as interpolated flaps, requiring a subsequent stage to inset the flap. Interpolated postauricular flaps have been described as a single-stage procedure, which is accomplished by de-epithelialization of the skin of the flap’s pedicle.15,24 A modification has also been described that consists of harvesting of the flap from the medial surface of the auricle to repair a skin defect of the lateral surface. The technique requires de-epithelialization of a portion of the pedicle.16
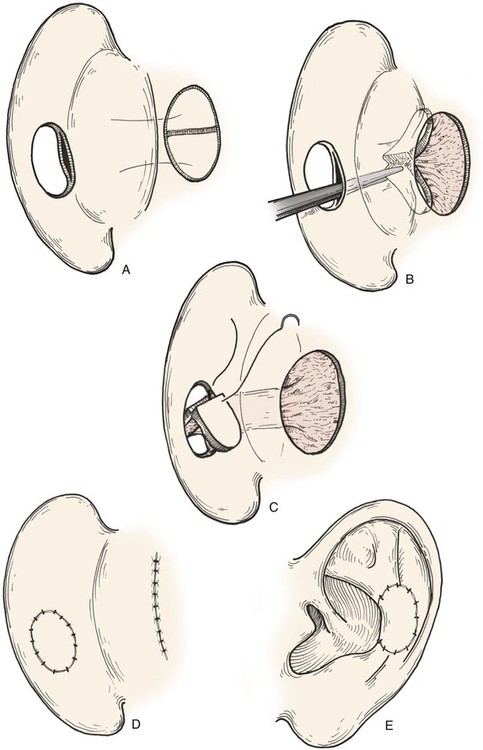
FIGURE 22-11 A, Subcutaneous tissue pedicled island advancement flap designed on postauricular skin. Flap is divided to provide medial and lateral surface cover for defect. B, Subcutaneous tissue pedicle tunneled beneath medial auricular skin. C, Flap folded on itself to provide skin cover of medial and lateral aspect of defect. D, E, Flap sutured in place. Donor defect closed.
The principles set forth by Tanzer and colleagues for microtia reconstruction are germane to reconstruction of large acquired auricular defects.26,27 A three-dimensional autologous rib cartilage framework and soft tissue coverage are the mainstays of microtia repair. Similar structural frameworks may be used for reconstruction of large full-thickness auricular defects. Success depends on the use of supple, thin, and highly vascular skin flaps to cover cartilage grafts and to ensure refined contour of the reconstructed ear. When local skin is scarred or absent, as is often the case with acquired defects, a temporoparietal fascia flap may provide the necessary thin, highly vascular covering for cartilage grafts and concomitantly serve as a recipient site for overlying split- or full-thickness skin grafting. When the vertical height of the ear is likely to diminish by more than 20 mm by use of a local flap, repair with a regional flap is more appropriate. The temporoparietal fascia flap, combined with autologous cartilage grafting to replace auricular framework deficiencies, has proved to be the most versatile regional flap for reconstruction of larger full-thickness ear defects.
Auricular Reconstruction Based on Anatomic Location
Defects of Conchal Bowl and Root of Helix
Conchal bowl defects may be allowed to heal by secondary intention, which usually results in minimal or no auricular distortion. In subtotal conchal bowl defects, full-thickness skin grafts provide adequate aesthetic results and permit easy postoperative monitoring of the area after extirpation of a cutaneous malignant neoplasm (Fig. 22-12). If exposed cartilage is devoid of perichondrium, it can be safely resected because conchal cartilage is not necessary for auricular form. The medial auricular skin becomes the nutrient bed for the skin graft. Graft immobilization is achieved with a bolster dressing.
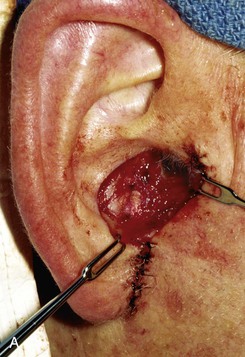
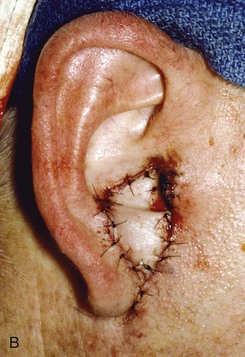
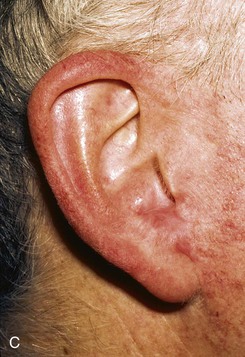
FIGURE 22-12 A, Full-thickness defect of concha cavum, with intact helical rim. B, Defect repaired with postauricular island advancement flap pedicled on postauricular muscle and subcutaneous tissue. Skin island folded to provide medial and lateral coverage. C, Postoperative result at 6 months.
A postauricular subcutaneous tissue pedicled island advancement flap is well suited for reconstruction of deep full-thickness conchal defects because of the flap’s proximity to the defect. This flap is an excellent choice />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses