Chapter 2
Development of the Head, Face, and Mouth
Joseph A. Grasso
Department of Cell Biology, School of Medicine, University of Connecticut
Introduction
Congenital facial and jaw defects represent a significant health problem worldwide, making it important for oral health professionals to understand the embryologic processes involved in facial and jaw development. While structural defects are conspicuous and gain clinical attention, developmental anomalies of the face and jaws often cause less visible functional problems, e.g., malocclusions, which challenge the oral health specialist.
The purpose of this chapter is to describe the morphological events leading to development of the face and jaws, including a brief description of early embryonic development. During the preceding decade, numerous studies have identified molecular and genetic factors involved in facial and jaw development, factors of the utmost importance to a comprehensive understanding of the development of this region. Although these factors are not addressed in this chapter, the reader is referred to several excellent reviews included in the list of references.
Early events establishing the head region
Formation of the germ layers: gastrulation
Fertilization, the union of male and female gametes, is followed by cleavage, a series of rapid cell divisions which give rise to the blastocyst. The blastocyst consists of an outer layer of trophoblast which surrounds a large cavity, or blastocoele, at one pole of which is located a cellular aggregation, the inner cell mass. (Cells of the inner cell mass of mammalian embryos, isolated and grown in culture, are called embryonic stem cells.) Early in the second week, cavitation in the inner cell mass forms a protective sac, or amnion. Simultaneously, delamination of the inner cell mass produces the bilaminar disk, which lies in and forms the floor of the amnion (Fig. 2.1). This flattened disk consists of an upper layer, the epiblast, which is continuous at its margins with the amnion, and a lower layer, or hypoblast, which grows circumferentially around the blastocoele to form the lining, or exocoelomic membrane, of the primitive yolk sac (Figs. 2.1a, 2.1b). The epiblast, a single layer of columnar cells, is the source of the three primary germ layers – ectoderm, mesoderm, and endoderm – which ultimately form the embryo. The trophoblast contributes to formation of the placenta.


Figure 2.1 Structural features and development of the blastocyst during weeks 1 and 2.
(Adapted from Hamilton and Mossman, 1972.)
Germ layer formation occurs during gastrulation, which begins early in the third week and is signaled by the appearance of the primitive streak. In addition to its role in formation of the germ layers, the primitive streak defines the polarity of the embryo: the cranial-caudal axis, the dorsal-ventral axis, medial and lateral sidedness, and the right and left axial halves of the embryo.
The primitive streak arises from epiblast cells which migrate toward the midline at the posterior end of the bilaminar disk, now the embryo. At the midline, these cells aggregate and invaginate to form a central furrow, or primitive groove, within the primitive streak (Fig. 2.2). As more epiblast cells move into the primitive streak, the primitive streak grows in length at its caudal end (Fig. 2.2a); at its cephalic end is a conspicuous bulge of epiblastic cells, the primitive node, whose center contains a depression, the primitive pit, which is continuous with the primitive groove (Fig. 2.2). The primitive streak and primitive node play key roles in the formation of the germ layers and the notochord, an axial structure of utmost importance in subsequent development. As formation of the germ layers and notochord progresses, the primitive streak is displaced caudally and gradually decreases in length (Figs. 2.2a, 2.2b). At the end of gastrulation, it is reduced to an insignificant aggregation of cells at the posterior end of the embryo.


Figure 2.2 Dorsal views of the embryo during week 2 showing the primitive streak (purple), its axial location, and movements of epiblast through the primitive streak. Dark arrows denote the movement of epiblast toward the primitive streak; the gray arrows indicate directions of movement of mesenchymal epiblast from the primitive streak to form the germ layers. Note decreased length of primitive streak as germ layers and notochord (green) develop.
The primitive streak provides a pathway through which cells of the epiblast invaginate to form endoderm and mesoderm. Dividing epiblast cells migrate to the primitive streak and sink into the primitive groove. During this migration, these epiblastic cells change shape and become motile cells, or mesenchyme, which detach and emerge from the primitive streak to enter the space below the epiblast.
Formation of the germ layers: the endoderm
Some of these mesenchymal cells, passing ventrally from the primitive streak, invade and intercalate between the cells of the underlying hypoblast to form endoderm, which displaces the hypoblast laterally into the extraembryonic area (Fig. 2.3). Growth of the newly formed endoderm at the expense of the displaced hypoblast results in the formation of the secondary yolk sac, a vesicle directly attached to the ventral body of the embryo and lined entirely by endoderm.

Figure 2.3 Cross-sectional view of embryo to show movements of epiblast through primitive streak to form the endoderm (orange) and intraembryonic mesoderm (red).
Though displaced by endoderm, the hypoblast contributes to formation of the extraembryonic mesoderm (Fig. 2.1b) within which blood islands and elements of the vascular system ultimately develop. In humans, a region of the hypoblast known as anterior visceral endoderm participates in formation of the prechordal plate, an important landmark in head development. The prechordal plate appears at the cranial pole of the embryo where endoderm becomes tightly adherent to the overlying epiblast (now called ectoderm) to form a thickened bilaminar plate (Figs. 2.6; 2.7a). The prechordal plate later becomes the oropharyngeal membrane which separates the embryonic mouth, or stomodeum, from the embryonic pharynx. Although a transient structure, the prechordal plate plays a significant role in development of the head region: it induces differentiation and bilateralization of the forebrain, defines the cranial axis of the embryo, and is a useful landmark in subsequent development of the oral cavity and pharynx.
Formation of the germ layers: intraembryonic mesoderm
The major proportion of invaginating epiblast migrates laterally and cranially, filling the region between the endoderm and overlying ectoderm to form the intraembryonic mesoderm (Figs. 2.2a, 2.2b; Fig. 2.3). At the margins of the embryo, this mesoderm is continuous with extraembryonic mesoderm (Fig. 2.3). As the forming intraembryonic mesoderm expands toward the cranial end of the embryo, it passes in front of the prechordal plate to meet the mesoderm of the opposite side, giving rise to a concentric, horseshoe-shaped mass of mesoderm within which will appear the cardiogenic plate that forms the heart (Fig. 2.6).
Formation of the notochord
As the germ layers develop, mesenchymal cells detach from the cranial end of the primitive node and migrate in the midline toward the anterior end of the embryo to eventually form a solid rod, or notochord (Fig. 2.2b). The notochord grows toward the cranial pole of the embryo where its further extension is impeded by the tightly adherent endoderm and ectoderm of the prechordal plate. Interposed between the tip of the notochord and the caudal edge of the prechordal plate is an area of mesoderm termed prechordal mesoderm which, augmented by paraxial mesoderm in the region near the midbrain, ultimately gives rise to the extraocular muscles.
The notochord provides axial support for the embryo. It interacts with the overlying ectoderm to induce differentiation of the neural tube which becomes the central nervous system. It influences organization of somites and development of the vertebral column. In an adult, it persists as the gelatinous central nucleus pulposus of the intervertebral disks.
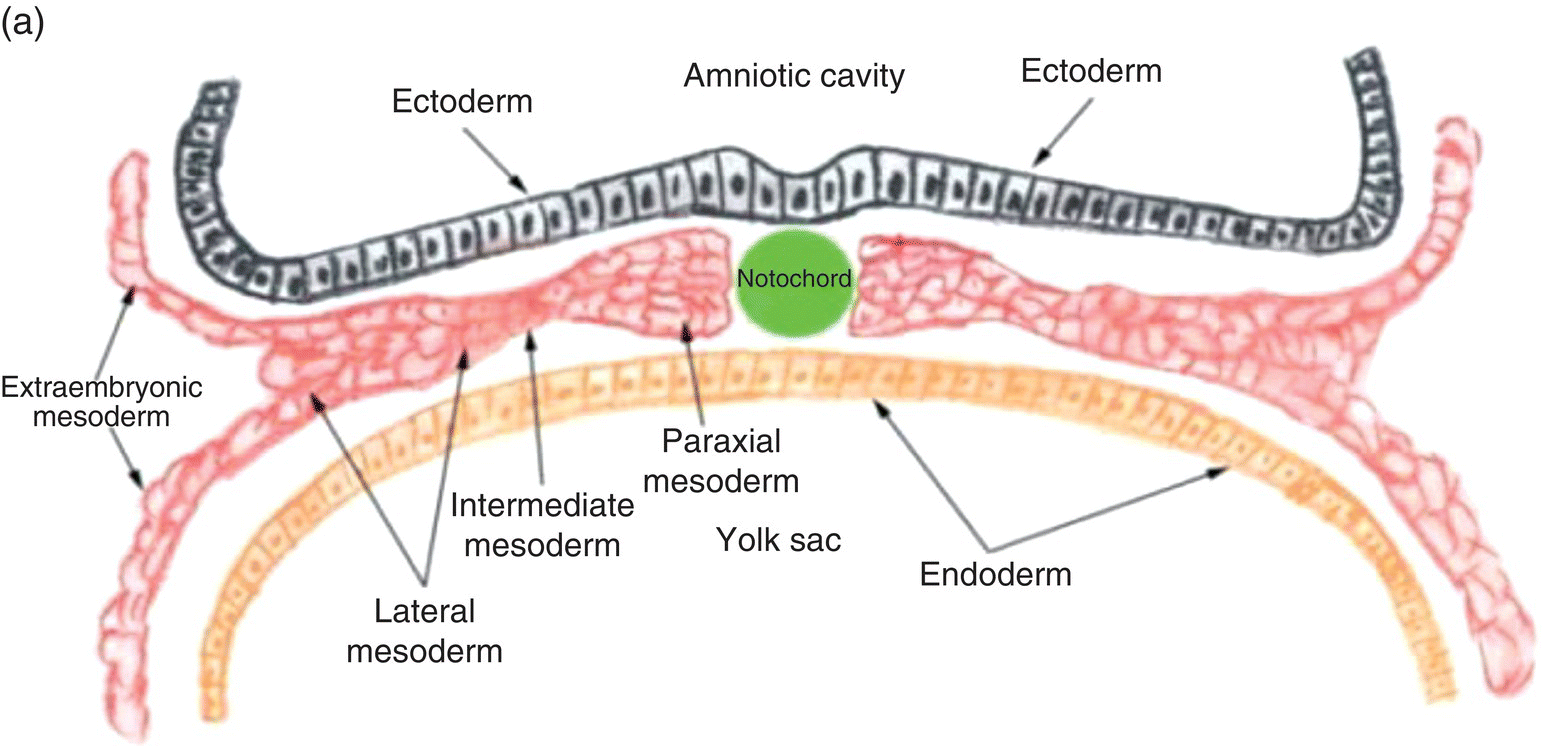
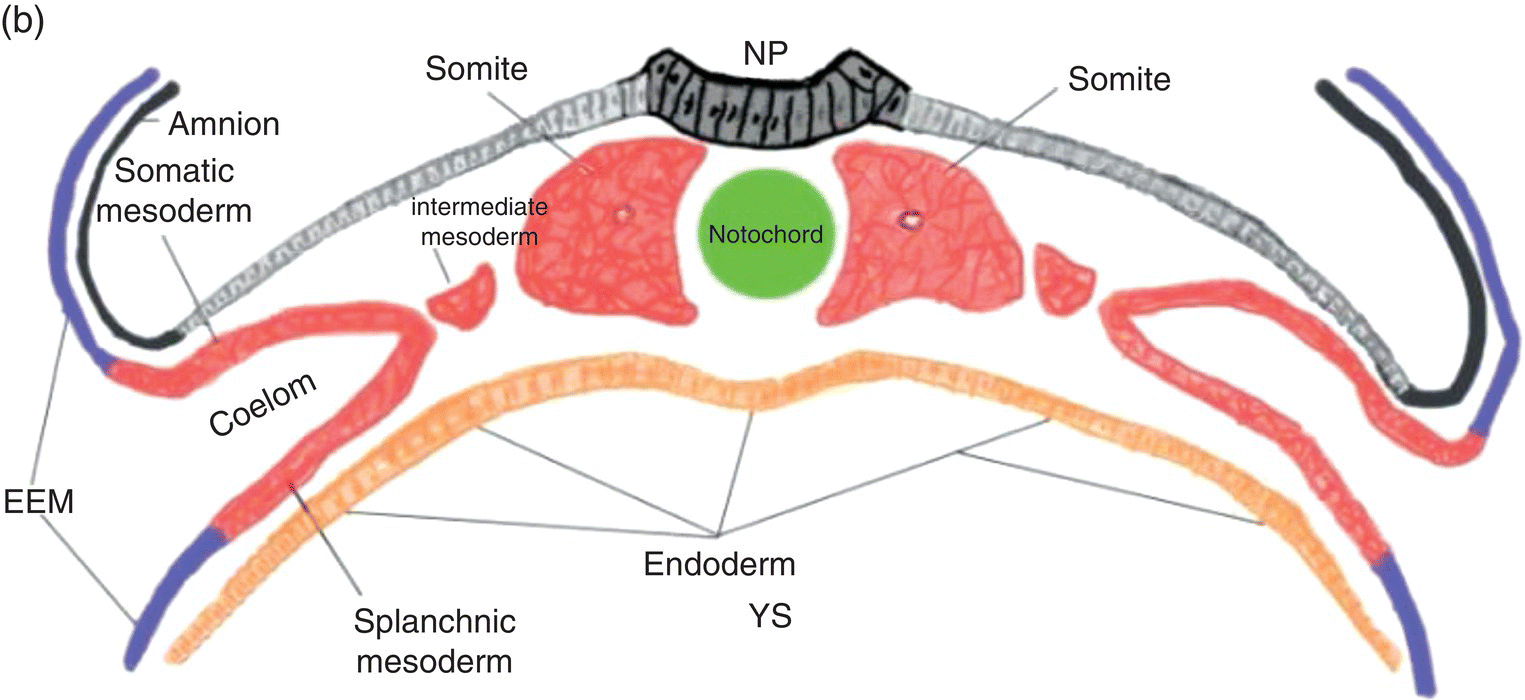
Figure 2.4 Transverse sections illustrating the organization of the three germ layers during week 4 (a) and the organization of intraembryonic mesoderm forming somites, intermediate, and lateral plate mesoderm during week 5 (b). YS, Yolk sac; NP, neural plate.
Regional differentiation of mesoderm: somite formation and specialization
Three specialized regions, each with different developmental fates, soon become evident within the intraembryonic mesoderm of the trunk region (Fig. 2.4a): (1) paraxial mesoderm, adjacent to the notochord; (2) lateral to the paraxial mesoderm, intermediate mesoderm, from which the kidneys, adrenal glands, and portions of the genitourinary tract are derived; and, (3) lateral plate mesoderm, which is continuous with the extraembryonic mesoderm at the margins of the embryo.
Beginning at 20 days, in the region of the embryo corresponding to the future occipital zone, the paraxial mesoderm undergoes segmentation to form somites, block-like segregated epithelioid aggregations of mesoderm (Fig. 2.4b; Fig. 2.6). Over the next 10 days, sequential segmentation of the paraxial mesoderm in a cranio-caudal wave produces 40 to 44 pairs of somites: 4 to 5 occipital; 8 cervical; 12 thoracic; 5 lumbar; 5 sacral; and 7 to 10 coccygeal. Each somite becomes associated with a specific nerve from the adjacent level of the developing spinal cord, an association responsible for the segmental pattern of the peripheral nervous system. Skeletal muscles and tissues derived from somites retain their associated spinal nerves throughout development and ultimately organize to form the body segments, or dermatomes, of the adult trunk. The somites and their associated nerves are responsible for the establishment of the adult metameric pattern visualized as the dermatomes of the neck and trunk.
Each somite displays three subdivisions: (1) a ventromedial sclerotome which will form the vertebrae and the vertebral column; (2) a dorsolateral dermatome which contributes to the dermis of the skin; and (3) an intermediate myotome which forms the skeletal muscles of the trunk and limbs.
The occipital somites contribute important structures to the adult head. Their sclerotome gives rise to the occipital bone of the skull; their myotome forms the muscles of the tongue.
The paraxial mesoderm cranial to the first occipital somite does not undergo segmentation or form somites. Unsegmented paraxial mesoderm constitutes the main proportion of head mesoderm. A major portion of it migrates into the embryonic pharynx to form pharyngeal (branchial) mesoderm, from which the branchiomotor skeletal muscles of the jaw, face, and neck are derived. It also forms certain bones of the skull: parietal bone, petrous portion of temporal bone, and sphenoid bones.
In the embryonic region destined to become the trunk, cavitation within the lateral plate mesoderm splits it (Fig. 2.4) into two layers: (1) a somatic layer that is associated with the overlying ectoderm and contributes to the formation of parietal serous membranes and connective tissue investments of trunk musculature; (2) a splanchnic layer that is associated with the endoderm and will form the outer layers of the digestive tract and the visceral serous membranes. The space formed by cavitation between the somatic and splanchnic layers of the lateral mesoderm coalesces to become the intraembryonic coelom, which eventually will be incorporated into the body of the embryo where it contributes to formation of the peritoneal, pleural, and pericardial cavities.
In the head region, the boundary between paraxial and lateral mesoderm is difficult to visualize because of the formation of the head fold (described below). Except for the lateral plate mesoderm that forms the cardiogenic plate, the lateral mesoderm of the head region probably merges with the cranial paraxial mesoderm to form the pharyngeal or branchial mesoderm (described below).
Induction of the neural tube and appearance of the brain
At 17 to 18 days, before the end of gastrulation, neurulation, the formation of the neural tube, is initiated by molecular and chemical induction of the overlying ectoderm by the notochord. In response to induction by the notochord, the ectoderm in the midline and immediate flanking regions thickens and increases in height to form the neural plate (Figs. 2.5a, 2.5b). Growth and shifts in position of neural plate cells elevate the sides of the neural plate to form neural folds between which lies a furrow, the neural groove (Fig. 2.5b). Continued proliferation, changes in shape and size, and the cytoskeleton of the neural plate cells work in tandem to increase the length, thickness, and depth of the neural folds. Simultaneously, extrinsic forces generated by the concomitant growth of the adjacent non-neural ectoderm assist to elevate the folds and deepen the neural groove. Finally, the dorsal leading edges of the neural folds approximate one another in the midline, detach from the overlying ectoderm, and merge to form the neural tube, which sinks into the body of the embryo (Figs. 2.5c, 2.5d). The overlying ectoderm coalesces as a continuous sheet of cutaneous ectoderm over the dorsal body surface.




Figure 2.5 (a–d) Steps in the formation of the neural tube and neural crest. The early subdivision of somites is also depicted (d).
(Modified from Hamilton and Mossman, 1972.)
Fusion of the neural folds to produce the neural tube begins in the region of the embryo corresponding to the future neck and proceeds from that point in a cranial and caudal direction. Both leading edges of the forming neural tube remain temporarily open at their cranial and caudal ends as the cranial and caudal neuropores, respectively, but eventually they close to produce a neural tube containing a central cavity, the neural canal. The neural canal will become the brain ventricles and spinal canal. Spina bifida results from failure of the neural folds to close, leaving the spinal cord and its canal in open contact with the external environment.
The neural tube expands and enlarges at its cranial end to form the brain, but remains tapered and narrowed caudally where it forms the spinal cord. The embryonic brain initially consists of three primary divisions but subsequent subdivision of the forebrain and hindbrain produces five segments from which the adult brain develops:

Transient segments termed neuromeres or rhombomeres appear in the hindbrain and are important in the subsequent formation of cranial nerves and the neural crest. In humans, seven rhombomeres appear, each designated by number (Rh1-Rh7).
Although development of the brain is beyond the scope of this chapter, growth and differentiation of the brain exert major influences on head and facial development. Its contributions to the ultimate form of the head region include the following.
- Brain growth and expansion induce formation of the head fold (see below) which leads to the emergence of the primitive gut and embryonic pharynx, a key primordium of facial and jaw development.
- Growth and bilateralization of the forebrain to form the cerebrum and cerebral hemispheres influences the ultimate shape and form of the head. Developmental failures affecting the forebrain can result in holoprosencephaly, which causes severe deformities in the shape and appearance of the head and face.
- Evagination of the diencephalon produces the optic vesicles and cups from which originate the retina and uvea of the eye. The optic vesicles induce formation of the lens placode from surface ectoderm. The placode forms the lens; neural crest from the forebrain region condenses to form the cornea.
- Establishment of appropriate central connections with the nasal (olfactory) and otic placodes whose differentiation from surface ectoderm elaborates the structures associated with the olfactory and auditory special senses, respectively.
- Formation and patterning of cranial nerves.
- Formation of the head neural crest, a component of primary significance in facial and jaw development.
Formation of the neural crest
Shortly before the neural folds close to form the neural tube, neuroectodermal cells at the dorsal lips of the neural folds differentiate to form the neural crest (Figs. 2.5b–d). Neural crest arises along the entire length of the neural tube, extending in sheets or cords along the dorsolateral margins of the neural tube, between the tube and surface ectoderm (Figs. 2.5c, 2.5d). Detaching from the neural tube, the neural crest migrates, either as individual or groups of motile cells or, more often, as sheets or clusters of contiguous cells, along specific routes which conduct it to appropriate destinations throughout the embryo.
Neural crest constitutes a pluripotential mesenchyme that is the source of diverse tissue types: neural, glial, skeletal, connective, pigment, and secretory. Neural crest-derived structures include craniospinal sensory and autonomic ganglia, the enteric nervous system, adrenal medulla, calcitonin-secreting cells of the thyroid, Schwann cells, dermal melanocytes, adipose cells, and the cardiac outflow tract.
In the head region, neural crest forms a major portion of the skull, maxilla, mandible, auditory ossicles, hyoid bone, and larynx. It contributes to development of the cranial sensory and parasympathetic autonomic ganglia, forms pigment retinal cells and smooth muscles of the eye, and contributes to formation of the cornea. It forms the dentin and cementum of the teeth, the periodontal ligament and alveolar bone that support the teeth, connective tissue stroma of salivary glands and of the tongue, the pia-arachnoid meninges of the brain, glial cells, and the carotid bodies and sinus. Neural crest is a primordial tissue of fundamental importance to embryonic development, especially development of the head, face, and jaws.
Formation of the body folds and the head region
The formation of the head fold is a key event in the development of the face and jaws. At the beginning of the fourth week, the embryo is a flattened disk located in the floor of the amnion and forming the roof of the secondary yolk sac (Fig. 2.7a). At the cranial end, the prechordal plate and the cardiogenic plate lie in front of the leading edge of the expanding neural tube (Figs. 2.6, 2.7a). The prechordal plate consists of a dorsal layer of ectoderm tightly bound to a ventral layer of endoderm (Fig. 2.7a). The heart has begun to form and, by day 23, begins to contract to assume its life-long role as a pump that propels blood throughout the embryo and body. Over the course of several days, differential growth at the cranial and caudal ends of the neural tube, as well as expansion of the amnion, will compel folding of the embryonic disk to form the head, tail, and lateral folds that transform the embryo from a flat disk into a cylindrical body tube.

Figure 2.6 Dorsal view of a human embryo prior to head fold formation. The neural plate (blue) is expanded at its cranial end and will form the brain. The cardiogenic plate (bright red) lies anterior to the prechordal plate. Top and transverse views of the somites (light red) are shown. The course of the intraembryonic coelom, the space between the somatic and splanchnic layers of lateral plate mesoderm, is shown by the dashed lines.
(Modified from Hamilton and Mossman, 1972.)




Figure 2.7 (a–d) Sagittal views showing the formation of the head (and tail) folds and the incorporation of the yolk sac (YS) into the body of the embryo to form the embryonic gut, whose subdivisions are labeled (c, d). Note the orientation of the prechordal plate (oropharyngeal membrane) as the head fold evolves; at 26-27 days, the oropharyngeal membrane ruptures to establish communication between the mouth and embryonic pharynx that arises from the foregut (c, d).
(Modified from Arey, 1968.)
The cranial end of the neural tube expands to form the forebrain, which grows and extends over the prechordal plate and the developing heart (Fig. 2.7b). Coupled with the simultaneous expansion of the amnion, the growth of the forebrain causes the cephalic end of the embryo to bend ventrally, using the prechordal plate (now called the oropharyngeal membrane) as a hinge, to form the head fold (Fig. 2.7b). Formation of the head fold brings the heart and the trailing oropharyngeal membrane below the developing brain into the ventral region of the embryo (Figs. 2.7b, 2.7c). Expansion of the amnion simultaneous with formation and expansion of the head fold results in lateral body folds such that the embryo constricts the yolk sac like a purse-string, absorbing the proximal region of the yolk sac into the body of the embryo (Figs. 2.7b–d).
The incorporated portion of the yolk sac lined by endoderm becomes the primitive gut which is connected to the yolk sac lying outside the embryo by means of the yolk stalk (Figs. 2.7a–d). Immediately above and directly continuous with the yolk stalk is the midgut; behind the region of attachment of the yolk stalk lies the hindgut. The cranial end, or foregut, extends forward above the heart to terminate at and behind the oropharyngeal membrane, which closes off the anterior portion of the gut (Figs. 2.7c, 2.7c).
The formation of the head fold has inverted the position of ectoderm and endoderm in the oropharyngeal membrane relative to their initial position (compare Fig. 2.7a with Figs. 2.7c, 2.7d) so that the ectoderm is now below while the endoderm is above and directly continuous with the endoderm of the foregut. Growth of the forebrain has produced a deep depression in the surface ectoderm, the embryonic mouth or stomodeum, which lies in front of and below the oropharyngeal membrane (Fig. 2.7c). The oropharyngeal membrane separates the stomodeum from the anterior region of the foregut which will become the pharynx (Fig. 2.7c). The oropharyngeal membrane marks the boundary between ectodermal and endodermal domains in the subsequent formation of the oral cavity and pharynx (Figs. 2.7c, 2.7d). With perforation and disappearance of the oropharyngeal membrane at 24 to 25 days, the stomodeum becomes continuous with and opens directly into the embryonic pharynx (Fig. 2.7d).
Development of the pharyngeal region and the pharyngeal arches
The developmental history of the pharyngeal arches is intimately linked to the formation of the face, jaws, oral cavity, and pharynx. Knowledge of the formation, organization, and fate of the pharyngeal arches helps in understanding the anatomy of the adult head and the etiology of congenital defects of the jaws and oral cavity. The pharyngeal arches are responsible for the segmental distribution of cranial nerves in the adult face and jaw, a pattern of segmentation that is analogous to the dermatomes of the trunk. This segmentation or metamerism arises from the association of a specific cranial nerve with particular pharyngeal arches, a relationship that persists in all derivatives of the arches.
Formation and organization of the pharyngeal arches
The formation of the pharynx and the pharyngeal arches is signaled by changes in the shape of the anterior region of the foregut. As it transforms into the pharynx, this region of the foregut becomes flattened dorsoventrally and expands to form a funnel-shaped tube (Figs. 2.8a–c). It is widest immediately behind the oropharyngeal membrane and narrows as it passes posteriorly to join the caudal part of the foregut (Figs. 2.8a–c). The pharyngeal arches form the lateral walls of the pharynx, appearing as swellings or elevations that develop sequentially in a cranio-caudal succession during the fourth week. The pharyngeal endoderm induces formation of the pharyngeal arches through its interactions with the other germ layers and with neural crest, and is the major determinant in the elaboration of the pharyngeal arch complex.



Figure 2.8 (a–c) The organization of the embryonic pharynx. Figure 8(a) is a dorsal view showing the pharyngeal arches and the pharyngeal floor. A schematic diagram of the pharyngeal arches shown in 8(a) is depicted in Figure 8(b). Figure 8(c) shows a sagittal section of the embryonic pharynx. Dashed lines indicate the original location of the oropharyngeal membrane. Germ layer composition is color coded.
(Panels (a) and (c) modified from Hamilton and Mossman, 1972.)
Five pairs of pharyngeal arches, numbered I–VI, ultimately appear in the human pharynx (the fifth either never appears or is rapidly absorbed into the other arches). The first and second pharyngeal arches are called the mandibular and hyoid arches, respectively, but the remaining arches are designated by number only. The boundaries of each arch are defined externally by invaginations of the surface ectoderm, the pharyngeal clefts or grooves, and internally by pharyngeal pouches, outpocketings of the endoderm lining the pharyngeal lumen (Figs. 2.8a–c). The pouches correspond precisely in position to the clefts or grooves, with the two closely approximating one another but separated by a thin membrane and never establishing continuity. The pouches and clefts separate each arch from the next and are designated by number, I-V, the first pharyngeal pouch (I) and cleft (I) separating the first from the second arch, etc. In fish and amphibians, rupture of the thin membrane separating the clefts from the pouches produces the gill slits.
The tissue mass occupying the area between the ectoderm and endoderm of each pharyngeal arch consists of a core of pharyngeal or branchial mesoderm which is surrounded by a ring of neural crest (Fig. 2.8b). The pharyngeal (branchial) mesoderm is derived primarily from cranial paraxial mesoderm which, together with neural crest, arise from corresponding levels adjacent to the hindbrain and migrate coordinately to their specific destination in the pharyngeal arch complex.
Coursing through the core of each pharyngeal arch is an aortic arch (Figs. 2.8a, 2.8b) which connects the truncus arteriosus, the outflow tract of the embryonic heart that is located below the pharynx (Fig. 2.8c), to the dorsal aorta in the dorsal body wall. Only aortic arches III, IV, and VI persist in the adult as significant vessels.
The roof of the pharynx consists of the dorsal body wall containing the neural tube and associated structures, while the floor lies directly above the forming heart (Fig. 2.8c). The floor and pharyngeal arches merge seamlessly to form ventrolateral ridges or bars whose developmental history and fate are linked and intimately coordinated during development of the jaws, oral cavity, and pharynx.
The rupture and disappearance of the oropharyngeal membrane between the pharynx and stomodeum at 25 to 26 days establishes continuity between the oral cavity and pharynx. Because of the extensive tissue migrations that occur during development of the oral cavity, tongue, and pharynx, it is difficult to delineate the original position of the oropharyngeal membrane. An imaginary line drawn from the foramen caecum and sulcus terminalis of the tongue upward toward the palate just in front of the tonsillar fossae roughly approximates its position in the adult.
Innervation of the pharyngeal arches
Each pharyngeal arch is innervated by a specific cranial nerve (Fig. 2.9), an association that is retained by all adult derivatives of the arch. Four cranial nerves provide the innervation of the pharyngeal arches: V, VII, IX, and X. These nerves contain both motor and sensory components and are, therefore, mixed nerves. They arise sequentially from successive levels of the mid- and hindbrain and contain functional modalities not found in other nerves. All four nerves transmit motor fibers that are designated branchiomotor or special visceral motor because the skeletal muscles they innervate are derived from pharyngeal mesoderm. In addition, cranial nerves VII, IX, and X mediate taste sensation and contain parasympathetic motor components.

Figure 2.9 Innervation of the pharyngeal arches and the neck region in the 4 to 5 week embryo.
(Modified from Hamilton and Mossman, 1972).
The cranial nerve distribution to the pharyngeal arches is as follows.
- Trigeminal (V) – the nerve of the first or mandibular arch. In addition to the first arch, the trigeminal innervates the frontonasal process and its derivatives, and exhibits three divisions: ophthalmic to the frontonasal process, maxillary to the maxillary process of the first arch, mandibular to the mandibular process of the first arch. It mediates general sensation and conveys branchiomotor fibers to muscles derived from the first arch mesoderm. It is the principal sensory nerve of the head in adults. Its sensory ganglion is the semilunar (trigeminal) ganglion.
- Facial (VII) – nerve of the second or hyoid arch. It conveys branchiomotor and parasympathetic fibers and mediates taste from the body of the tongue. Its pretrematic branch to the mandibular arch becomes the chorda tympani, which conveys taste and parasympathetic fibers. Its sensory ganglion is the geniculate ganglion.
- Glossopharyngeal (IX) – nerve of the third arch. It mediates general sensation from wide areas of the adult pharynx, middle ear, and root of the tongue and taste from the latter. It contains two sensory ganglia, the superior and inferior (petrosal) ganglia. It conveys parasympathetic fibers to the parotid gland and branchiomotor to a single muscle, the stylopharyngeus.
- Vagus (X) – nerve of arches IV and VI. It mediates general sensation from the pharynx, larynx, and many thoracic and abdominal viscera. It provides some taste sensation from the root of the tongue and taste buds in the neonate pharynx. It conveys branchiomotor fibers to laryngeal and pharyngeal musculature and parasympathetic fibers to many viscera. It contains two sensory ganglia, the superior (jugular) and inferior (nodosal) ganglia.
Factors responsible for innervation pattern of the pharyngeal arches
The association between a pharyngeal arch and its cranial nerve supply is established early in development. As the cranial paraxial mesoderm forms alongside the hindbrain, it is innervated by nerve fibers emanating from corresponding levels of the adjacent hindbrain. When this paraxial mesoderm migrates to its specified pharyngeal arch to form pharyngeal mesoderm, it carries along its innervation. These nerve fibers become motor fibers which provide motor innervation to the skeletal muscles derived from the pharyngeal mesoderm.
Sensory innervation of each pharyngeal arch and its derivatives is derived from distinct streams of neural crest and the epibranchial (epipharyngeal) placodes. The latter are specializations of surface ectoderm that are induced by the pharyngeal endoderm and are located above and behind the pharyngeal clefts of the second, third, and fourth arches. Together with the epibranchial placodes, the neural crest streams establish the sensory innervation of the pharyngeal arches and form the cranial sensory ganglia. The more proximal ganglia, or segments of ganglia, arise from neural crest, the more distal ganglia, or segments, from epibranchial placodes. Cranial neural crest is also responsible for elaboration of the autonomic ganglia and autonomic connections that occur in the adult head region.
Each arch receives a separate, distinct stream of neural crest that arises from the hindbrain at the same level as the nerve fibers which innervate the paraxial mesoderm (Fig. 2.10). The neural crest stream that migrates into the first arch is the trigeminal crest which originates from the first and second rhombomeres (Rh1, Rh2) of the hindbrain, close to the origin of the trigeminal nerve (V). The mesencephalic crest, considered part of the trigeminal crest, migrates as frontonasal mesenchyme into the frontonasal region where it forms skeletal and neural elements of the frontal and nasal regions. The mesencephalic crest also contributes the main proportion of the trigeminal or semilunar ganglion and the ophthalmic division (V). The maxillary and mandibular divisions (V) are derived from trigeminal crest.

Figure 2.10 Origins and destination of the head neural crest streams.
(Adapted from Carlson, Human Embryology and Developmental Biology, 2009.)
Separate streams of neural crest course into the other pharyngeal arches (Fig. 2.10). The hyoid or facial crest originates from the fourth rhombomere (Rh4) of the hindbrain and migrates into the second arch. Behind the otic placode, the glossopharyngeal (Rh6) crest is directed into the third arch, and a part of the vagal (Rh7; C1–C5) crest stream passes into the fourth and fifth arch (Fig. 2.10
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


