Dental Implants
Fritz Finzen, Dorothy A. Perry, Based on the original work by
and Kamran Haghighat
• Describe the common types of dental implants.
• Discuss the indications and contraindications for dental implant therapy.
• Explain why titanium is the best biomaterial available for use in dental implants.
• Define the concept of osseointegration.
• Compare and contrast the bone and soft tissue interfaces of implants and the natural dentition.
• List the criteria for success used in implant therapy.
• Describe the maintenance protocol for implant patients.
• Evaluate the elements of appropriate home care regimens for patients with implants.
A multitude of dental implant systems are currently used in the rehabilitation of edentulous and partially dentate patients. The majority of the implants used in clinical dentistry are the root form type, endosseous implants, which are based on the principle of osseointegration. The term osseointegration was coined by Professor P.I. Bränemark to describe an intimate lattice that is formed between titanium implant surfaces and bone. From the late 1960s to the mid-1970s, dental implant systems were not osseointegrated and were not viewed favorably by the community because they were considered unpredictable. The observations and findings of Bränemark, Albrektsson, and colleagues, together with the long-term survival data of osseointegrated dental implants, have changed this perception so that the procedure has become a highly predictable and valuable option in the replacement of missing teeth.1–4
Today, in excess of 50 implant systems are available, with innovations being proposed and instituted by different manufacturers through intense competition. However, many of these newer systems lack the longitudinal research necessary before patient application. Ideally, longitudinal trials of 5 years or longer are required to forecast the validity of emerging treatment concepts adequately. The American Dental Association provides an acceptance program for endosseous implants through its Council on Dental Materials, Instruments, and Devices.5 Accepted implants have shown success for a minimum of 5 years in clinical trials of 50 or more patients.
Types of Implants
Subperiosteal Implants
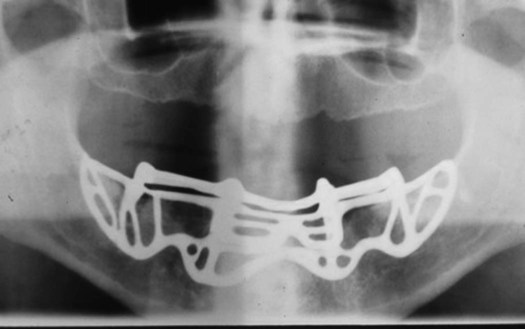
The subperiosteal implant is a custom-made cast framework that is placed beneath the periosteum over the alveolar bone. It can be used in either the maxilla or mandible. The frame rests on the jawbone, with no evidence of direct union with the bone in most cases. Posts of varying number, based on the prosthetic design, protrude through the soft tissues to provide anchorage for the denture or fixed prosthesis. A radiograph of a subperiosteal implant is presented in Figure 15-1.
Endosseous Implants
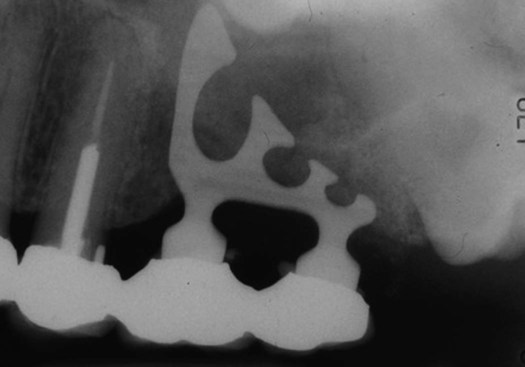
Endosseous implants, which come in a variety of different shapes, are placed within bone. They are broadly divided into blade and root form types. The root form variety is either screw-or cylinder-shaped, with different lengths, diameters, and manufacturer-specific design characteristics. The blade implant is no longer used because it has a high incidence of complications and failures. An example of a blade implant is shown in Figure 15-3.
Root form endosseous implants provide direct osseous anchorage through formation of an intimate lattice between the implant surface and bone. They are the most predictable and acceptable implant type used in clinical practice. These implants are used extensively for replacing missing teeth in partially and totally edentulous patients. Examples of several root form endosseous implants are presented in Figure 15-4.
Osseointegration
The definition of osseointegration has evolved through the development of more refined methods of studying the interface between the implant and surrounding bone. The precise nature of this integration is not fully understood. Originally, because of limitations in histologic techniques at the light microscope level, the term was defined as a direct implant to bone union without any intervening soft connective tissue,3,4 a condition that resembles that of functional ankylosis.6 A light microscopic view of osseointegration is presented in Figure 15-5. With the advent of scanning electron microscopy, more direct analysis of the interface is possible and osseointegration is more clearly understood. Scanning electron microscopy of the interface reveals a narrow nonmineralized zone, approximately 20 to 40 nm, between the bone and the implant, containing chondroitin sulfate glycosaminoglycans.7
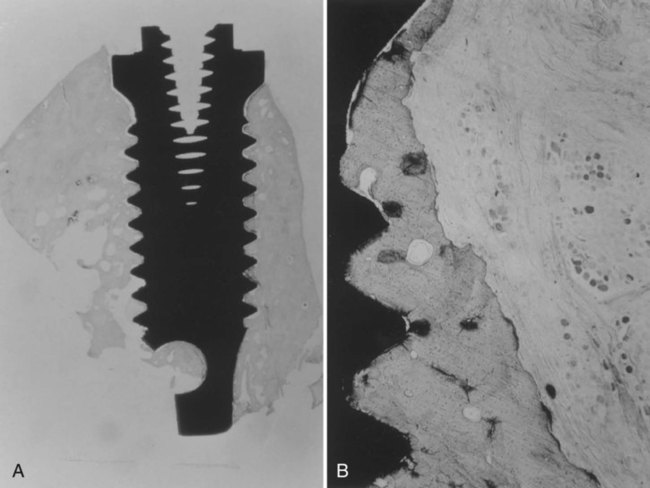
A, View of the root form with bone growth surrounding the titanium. B, Higher magnification showing bone apposition directly next to the titanium surface. (Used with permission from Bernard G, Carranza FA, Jovanovic S. Biologic aspects of dental implants. In: Newman MG, Takei HG, Carranza FA, eds. Clinical Periodontology. 9th ed. Philadelphia, PA: WB Saunders; 2002.)
The definition of osseointegration, as proposed by Bränemark, is a “direct structural and functional connection between ordered, living bone and the surface of a load-bearing implant.”8 This definition is based on clinical and radiographic implant stability rather than true interfacial arrangement, as observed histologically. Because implant integration involves soft and hard tissues, the term stably integrated implant has been suggested to describe implant integration better.9
Longitudinal observation of the bone-implant interface has also demonstrated that because of the dynamic nature of bone, 100% integration never develops, and the bone to implant contact is both time-dependent and influenced by implant surface characteristics. The amount of bone to implant contact varies among different implant systems (because of surface characteristics) and ranges from 30% to 70%.10 However, the exact amount of bone to implant contact required for success has not been determined.
The biologic processes involved in attainment and maintenance of implant integration depend on factors that include biomaterials and biocompatibility, implant design (e.g., length, diameter, shape, surface), bone factors, and surgical and loading considerations.2
Biocompatibility
Biocompatibility of a material is defined as allowing “close contact of living cells at its surface, which does not contain leachables (molecules that separate off the surface) that produce inflammation and which does not prevent growth and division of cells in culture.”11 Biocompatible materials are called biomaterials. Many different types of materials are considered biomaterials, including gold, stainless steel, cobalt-chromium alloys, bioactive glasses, niobium, hydroxyapatite, tricalcium phosphate, polymers, zirconium, and titanium. However, not all are compatible as an implant material.
Implant Design And Surface Conditions
Length
The range of implant lengths varies among manufacturers. Most conventionally used implants are between 8 and 14 mm long; this range conforms to natural root lengths. Selection is based on the available bone height at the implant site and proximity to vital structures, such as nerve trunks and blood vessels.12
Surface
Since the introduction of the original machined-surface implants, a variety of surface topographies and thread designs have been introduced. The initial stability of the implant is, in part, dependent on the surface texture.13 The rate of bone apposition and growth and the amount of bone to implant contact are influenced by implant surface characteristics. Many studies have demonstrated that a higher bone to implant contact is attained around rough-surfaced implants. Methods for producing roughened implant surfaces include grit blasting, acid etching, plasma spraying, and using additive surfaces such as hydroxyapatite coating. An increased rate of bone growth and amount of surface contact with bone allows for better transfer of forces to bone, facilitates earlier loading protocols (placement of restorations on the implants), and permits better success in areas with poorer bone quality. Biomaterials research has been underway to develop surface modifications to improve bone inductive characteristics and to make these treatments even more predictable.
State Of Host Site And Bone Factors
The amount of bone to implant contact achieved at the time of implant placement is related to the quantity and quality of bone and determines fixture stability. There is variability in the amount of cortical and cancellous tissue within the arch and between the maxilla and mandible. The volume density of cortical bone is three to four times that of cancellous bone. Cancellous bone therefore contributes less to implant stability at placement. Resorption of the alveolus is a natural sequelae to tooth loss. The extent of the resorptive process is dependent on the history of trauma or infection, the length of time since the loss occurred, and loading by removable prostheses. The quality of bone is also influenced by systemic conditions, such as osteoporosis, and social factors, such as smoking. Therefore, the shape and quality of bone must be considered when planning for implant therapy.14
Loading Considerations
There are no fixed guidelines for the length of healing time after surgery and before prosthetic loading of implants. Bränemark originally advised 3 months for the mandible and 6 months for the maxilla. The difference in healing times reflects the variation in bone characteristics.4 Movement of the implant more than 100 µm during the healing phase may result in fibrous tissue encapsulation of the implant rather than osseointegration, a result that does not provide long-term functional occlusal load. In cases in which the functional load can be controlled and the implant is determined as stable at the time of placement, immediate loading (placement of a restoration at the time of the implant surgery) is compatible with attaining osseointegration.15
Indications and Contraindications for Implant Therapy
Indications
The clinical situations in which osseointegrated implant-retained prostheses are used have expanded enormously. A diverse range of scenarios with varying degrees of complexity can be managed with the use of implants. These include the replacement of a single-tooth, treatment of partially and completely edentulous patients,16 and correction of maxillofacial deformities. Single-tooth replacement with an implant-supported prosthesis is considered to be a highly predictable and effective alternative to conventional prosthodontics procedures, such as fixed partial dentures (bridges), which often require preparation of healthy neighboring teeth. Specific indications for dental implants may include the following:
• Treatment of patients with strong gag reflexes to eliminate palatal coverage by removable prostheses
• Free end removable partial dentures
• Alternative to periodontally compromised teeth for bridge abutments
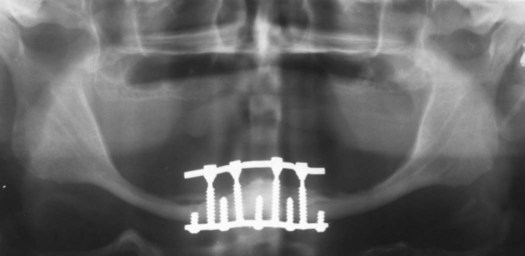
Examples of implant-supported prostheses are presented in Figures 15-6 through 15-9.
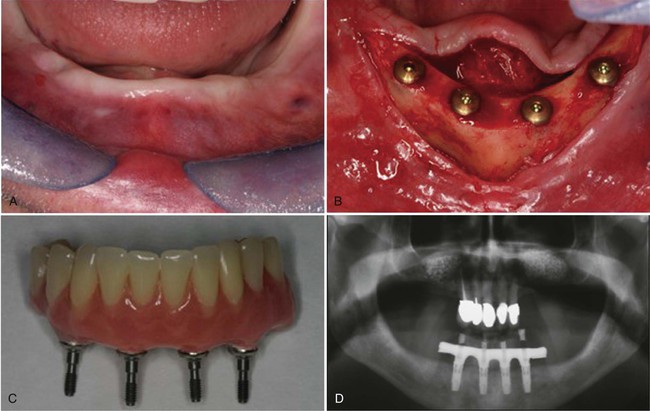
A, The mandibular ridge as it healed after implant placement. B, The flaps reflected to expose the implants. C, The denture with abutments to attach to the implants. D, Panographic radiograph of the denture in place, attached to the implant fixtures. Note the restored natural teeth in the maxillary arch.
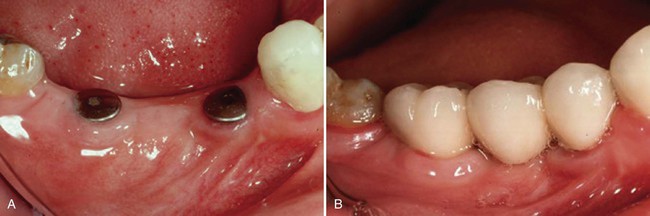
A, The implant fixtures. B, The restoration in place when the implants are loaded.
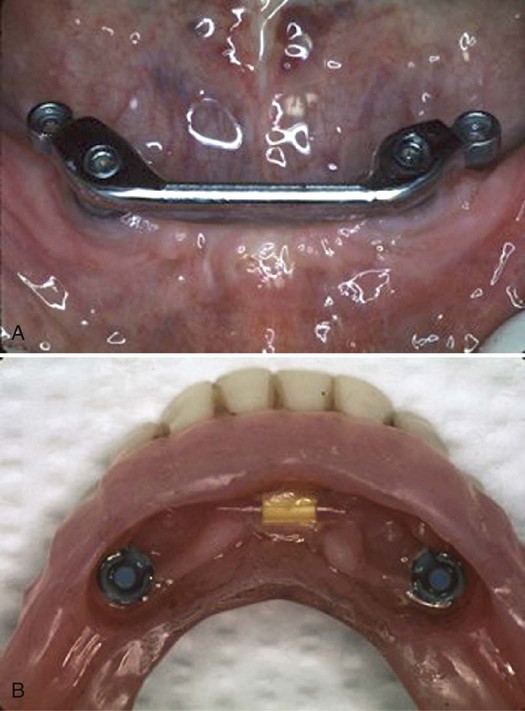
A, Two implants have been placed connected by a bar. B, The undersurface of the denture has attachments for connecting to the bar.
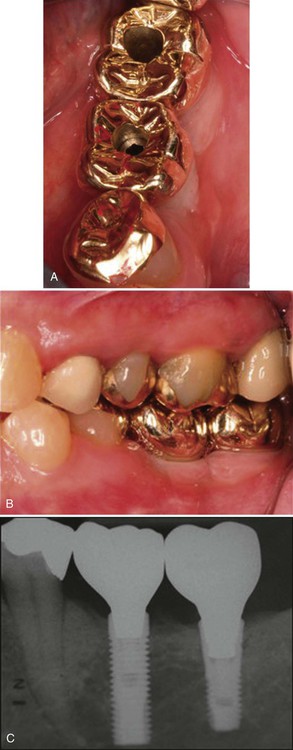
A, The crowns in place on the fixtures; the holes are access for the screws that attach the restoration to the abutment. B, Lateral view of the crowns in place. C, Radiographic view of the implant-supported crowns.
Contraindications
It has been speculated that the presence of certain systemic, local, and social factors may affect the outcome of therapy. Age is not a contraindication for dental implants. Treatment should be delayed in younger patients until growth is near completion because, unlike the natural dentition, implants remain stationary during dentoalveolar growth. Similarly, increasing age in older persons has no adverse effect on osseointegration as long as associated medical conditions are well controlled or modified.16,17
Osteoporosis, an age-related disease characterized by decreased bone mass and increased susceptibility to fractures, affects 20 million Americans, 80% of whom are older women. Hormone replacement therapy and osteoporosis do not appear to influence implant survival in this population.16,18 Therapy with bisphosphonates has recently been attributed to an increased risk of jaw osteonecrosis and patients who have been treated with IV bisphosphonates are particularly at risk. Consultation with the patient’s physician is recommended before implant therapy for patients receiving anticoagulant therapy because they are at risk for hemorrhage during surgical procedures. Patients on steroid therapy may have steroid-induced complications.16
The microbial pathogens associated with periodontal disease around natural teeth are also associated with disease progression around dental implants. Pathogenic bacteria associated with sites that exhibit active disease around teeth in partially dentate patients can colonize the peri-implant tissues.19–21 Effective treatment and control of any periodontal disease before implant therapy is essential, and implants should only be considered in patients who demonstrate commitment to good home care and maintenance routines.
The adverse effects of smoking on osseointegration and implant survival have been shown in many studies. Although smoking is not an absolute contraindication to implant therapy, it increases the risk of peri-implantitis and implant failure.16 A smoking cessation protocol before implant surgery and during the healing period improves the treatment outcome.
Teeth and Implants
The peri-implant soft tissue is shaped after abutment connection or, in the case of single-stage implant systems, forms around the transmucosal portion of the fixture (the section that extends from the bone into the oral cavity). The collagen fibers are aligned parallel to and organized in a circular arrangement around the supracrestal portion of the implant.22 There is no cemental layer over the implant surface, so fiber insertion is not possible. Initial observations by Gould and colleagues,23 and many subsequent studies, have demonstrated that peri-implant soft tissue adheres to the titanium collar of the implant by a hemidesmosomal and basal lamina attachment mechanism, analogous to that of the JE to enamel attachment.1
Implants acquire their stability and anchorage through osseointegration. There is no PDL so proprioceptive feedback is minimal, although proprioceptive mechanisms in the surrounding hard and soft tissues exist. Once osseointegrated, orthodontic movement of implants is not possible, and it is important to control the forces applied to implants for maintenance of the integrated interface. Unfavorable loading of implants is one etiologic factor for bone loss around implants.10
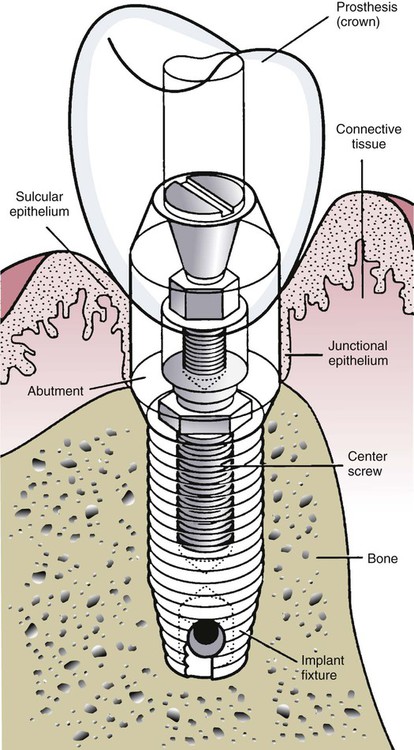
The biologic width around teeth—that is the dimension of the epithelial and connective tissue attachments—is approximately 2 mm. A similar implant biologic width has also been described for the peri-implant mucosa, which comprises a 2-mm long JE and a 1-mm zone of connective tissue.16 The connective tissue zone is poorly organized and exists between the JE, which is typically attached to the prosthetic component, called the implant abutment, and bone. The implant abutment extends from the implant through the soft tissues (transmucosally) and can be temporary (to allow soft tissue healing) or permanent. The implant abutment is connected to the implant fixture (the root analogue device in the bone) by an abutment screw.
The osseous crest around natural teeth in health follows the outline of the cementoenamel junction at a distance of 1 to 2 mm apically on the root surface. The location of the crestal bone level around implants depends on the implant design and may vary from 0.5 to 3 mm from the top of the implant fixture. These characteristics are compared in Table 15-1 and demonstrated diagrammatically in Figure 15-10.
TABLE 15-1
Comparison of Teeth and Implants in Health
| TEETH | IMPLANTS | |
| Gingival sulcus depth | Shallow | Depth dependent on implant type and prosthetic component length |
| Gingival fibers | Inserted into supracrestal root cementum | Fibers arranged parallel to implant |
| Location of crestal bone | 1-2 mm from cementoenamel junction | Dependent on implant design; ranges 0.5-2.5 mm from implant shoulder or to first thread |
| Connective tissue attachment | Sharpey’s collagen fibers inserted into alveolar bone and cementum | Bone-implant interface has no fiber insertions; filled with chondroitin sulfate glycosaminoglycans |
| Mobility | Physiologic as a function of PDL | No PDL; rigid fixation similar to that of functional ankylosis |
| Proprioception | Receptors within PDL | No receptors within interface |
Success Criteria
The clinical success of implant therapy is assessed by radiographic imaging, evaluation of implant mobility, and observing the surrounding soft tissue. Refinements to these conventional techniques include the use of digital and subtraction radiography, computed tomography, and devices that record the implant interface contact. Although evaluation of implants includes assessment of soft tissue parameters, such as probing depths, this criterion is considered to be of limited value around implants and remains controversial.1 Criteria commonly used to determine implant success are outlined in Box 15-1.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses