Fig. 4.1
Incomplete healing (scar tissue). (a) Surgical bony defect after endodontic surgery. (b) Reduction in size of a periapical bony defect by bone formation from periphery. Note the irregular border of the defect. (c) Further reduction in size of the periapical bony defect. Note that the residual defect is not associated with the root
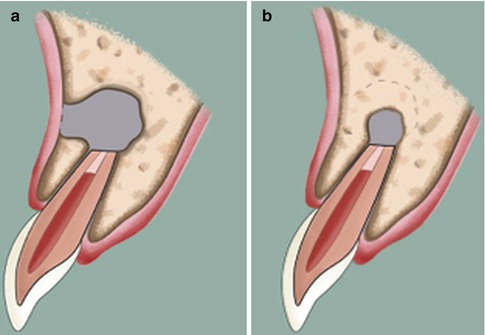
Fig. 4.2
Uncertain healing. (a) Surgical bony defect after endodontic surgery. (b) Reduction in size of a periapical bony defect by bone formation from periphery. Note that the size of the bony defect is larger than twice the width of normal periodontal ligament space
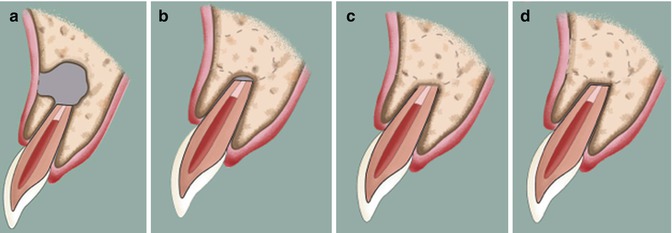
Fig. 4.3
Complete healing. (a) Surgical bony defect after endodontic surgery. (b) Significant reduction in size of a periapical bony defect by bone formation from periphery. Note that the size of the bony defect is no greater than twice the width of normal periodontal ligament space. (c) Complete bone fill without reestablishment of normal periodontal ligament space. (d) Complete bone fill with reconstitution of the periodontal ligament and lamina dura
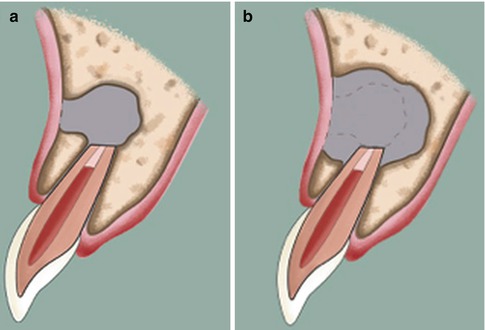
Fig. 4.4
Unsatisfactory healing. (a) Surgical bony defect after endodontic surgery. (b) Increase in size of a periapical bony defect by bone resorption in periphery
Due to the dynamic nature of wound healing, clinicians should be aware that postoperative diagnoses might change in some cases at different follow-up time points. Rud et al. [15] showed that based on the observation of 1,000 endodontic surgery cases, more than half of the cases with incomplete healing and uncertain healing underwent changes into other healing groups while almost all cases with complete healing and unsatisfactory healing remained unchanged. Notably, it was observed that these changes occurred mainly during the first year after the surgery and remained stable after the 4-year follow-up [15]. Therefore, clinicians are advised to have a 1-year follow-up as the first time point for outcome assessment and also recommended to have follow-ups up to 4 years in cases of uncertain healing before any further treatment is considered.
Etiology of Posttreatment Endodontic Diseases
The key to success of endodontic surgery is to identify and eliminate the origins or causes of apical periodontitis, which could not be addressed by nonsurgical root canal treatment. They include intraradicular infection and extraradicular etiologies.
Can Intraradicular Infection Be Successfully Treated by Root-End Surgery?
Posttreatment endodontic diseases caused by intraradicular infection in most cases can be treated by nonsurgical endodontic re-treatment. Despite technical advances in our disinfection armamentariums, anatomically complex areas in root canals such as isthmuses and fins that potentially harbor pathogenic microorganisms are not easily accessible due to the limitations of our instrumentation techniques during nonsurgical therapy [16–20]. Iatrogenic errors such as canal transportation and separated instruments that impair thorough root canal disinfection may be another significant factor contributing to failure of nonsurgical re-treatment [21, 22].
While endodontic surgery provides less opportunity to disinfect the entire root canal system than nonsurgical root canal treatment, in clinical situations where sufficient intracanal preparation and sealing cannot be obtained, endodontic surgery is a preferred alternative for teeth with apical periodontitis.
Can Extraradicular Etiologies Be Successfully Eliminated by Root-End Surgery?
If the source of infection or the cause of apical periodontitis resides in extraradicular areas [23–29], endodontic surgery is essential for healing. The strategy to cure apical periodontitis in these situations is rather straightforward. The elimination of the origin or cause of the periapical lesion by surgical debridement can lead to the resolution of apical periodontitis.
With the introduction of molecular methods to detect the microorganisms, a growing body of evidence exists to support the view that microorganisms exist in the extraradicular space [30, 31]. The existence of biofilms on the extraradicular surfaces also has been clearly demonstrated in microscopic observational studies [27, 28]. Biofilms can have up to 1,000 times more resistance than their planktonic counterpart [32–34]. Foreign materials beyond the apical foramen may also cause persistent apical periodontitis [35]. Lentil beans [36, 37], cellulose from paper points and cotton wool [38, 39], and small gutta-percha particles [40] may induce chronic inflammatory reaction and foreign body reaction because these materials are not easily degraded by host immune cells [35, 41]. Cholesterols derived from dead host blood and immune cells and plasma lipids [42, 43] are endogenously induced but also cause similar inflammatory reaction and result in persistent apical periodontitis (Fig. 4.1) [44, 45].
The incidence of cysts in periapical pathosis varies greatly between 6 and 54 % [46–50], and it is generally agreed that cystic lesions are less likely to be resolved by nonsurgical endodontic treatment [50–52]. A pocket cyst (bay cyst) where the cyst lumen is continuous with the root canal may resolve with nonsurgical root canal therapy [50, 53, 54]. On the other hand, a true cyst, which is not associated with root canals and is self-sustaining, is less likely to heal by nonsurgical endodontic treatment [50, 53, 54].
Wound Healing After Endodontic Surgery
The knowledge of wound healing processes after endodontic surgery is fundamental to our understanding and evaluation of outcomes, because the outcomes are considered as clinical reflections of wound healing. Harrison and Jurosky [55–57] described soft and hard tissue healing in three basic types of surgical wounds including incisional, dissectional, and excisional osseous wounds based on the histological observations of surgical wound healing in rhesus monkeys. In incisional wounds involving flap tissues, fibrin clot formation was observed at day 1, multilayered epithelial tissue formation at day 2, granulation tissue formation involving collagen synthesis by fibroblasts at days 3–4, and subsequent replacement of granulation tissues with fibrous connective tissues at days 14–28 [55]. Dissectional wounds including mucoperiosteal tissues and cortical bones also showed chronologically similar wound healing processes. The formation of fibrin clots occurred at day 1, granulation tissues were formed at days 3–4, and a new periosteum along the cortical bones and fibrous connective tissues was observed at day 14–28 [56]. In the dissectional wounds, degenerative changes in the cortical bones were closely associated with the absence of periosteal tissues, suggesting a protective effect of the periosteum on cortical bone necrosis [56]. In excisional osseous wounds, the osteotomy site was filled with coagulums at day 1 and replaced by granulation tissues originating from peripheral endosteal tissues at day 4 [57]. At day 14, most of the wound site was occupied with endosteal tissues and newly formed woven bones, which were in contact with dense fibrous connective tissues demarcating between the wound site and the overlying flap tissues [57]. These dense fibrous tissues were thought to participate in the periosteum reformation [57]. At day 28, increased maturation of woven bones and osteoid deposition on the surfaces of both cortical and trabecular bones were observed [57]. Clinicians should note that these healing events are expected to occur in surgical wound sites only if the etiological factors attributing to the posttreatment endodontic diseases are addressed.
Technical Differences and Their Implications for Outcome
The scientific support of outcome variations in endodontic surgery can be found in the different techniques employed in achieving disinfection and attaining an apical seal. Table 4.1 shows the main technical differences between TES and EMS and their implications.
Table 4.1
Technical differences between TES and EMS and their implications
|
Surgical procedure
|
TES
|
EMS
|
Implications
|
|---|---|---|---|
|
Osteotomy
|
Larger size
|
Smaller size
|
Healing time
|
|
Type of wound healing (repairvs. regeneration)
|
|||
|
Periodontal involvement
|
|||
|
Root-end resection
|
Greater bevel (~45°)
|
Minimal bevel (0–10°)
|
Removal of etiological factors
|
|
Greater resection (>3 mm)
|
Minimal (~3 mm) resection
|
Tooth stability
|
|
|
Retrogradepreparation
|
Deficient inspection ofresected root surface
|
Thorough inspection ofresected root surface
|
Removal of etiological factors
|
|
Altering original rootcanal morphology
|
Respecting original rootcanal morphology
|
||
|
Improper canal cleaning
|
Adequate canal cleaning
|
||
|
Root-end filling
|
No or inadequate apical seal
|
Adequate apical seal
|
Entombment of microorganisms
|
In reviewing these differences, the following definitions will be used, which are generally adopted in the majority of outcome studies [6, 58, 59]. The techniques of TES include root-end preparation with burs and root-end filling with amalgam or zinc oxide eugenol or CavitTM or gutta-percha without the use of the endoscope or microscope [15, 60–82]. In many instances, TES is also performed without any root-end filling materials [15, 62, 63, 65, 67, 68, 70, 72, 76, 79, 82]. By contrast, EMS techniques include root-end preparation with ultrasonic or sonic tips and root-end filling with IRM or EBA or MTA with the use of the endoscope or microscope [2, 83–91]. Due to insufficient or lack of magnification, TES requires a larger osteotomy than EMS to locate the periapical lesion and resect the root which may influence the progress of healing [83]. In general, both TES and EMS eliminate extraradicular etiologies by similar surgical debridement techniques; however, great technical differences exist between TES and EMS in addressing the intraradicular infection. An adequate apical seal with root-end filling materials is critical to a long-term success of the root-end surgery. The root-end filling materials used in TES such as amalgam or temporary filling materials (zinc oxide eugenol or CavitTM) or burnished gutta-percha are inferior to those in EMS – MTA, EBA, IRM – in their sealing ability and biocompatibility [92–96]. Therefore, it is not surprising to see a gradual deterioration and relapse of apical periodontitis in initially healed cases after TES, leading to remarkable outcome differences between EMS and TES in long-term success.
Outcome Variations of Endodontic Surgery
The technical advantages conferred by EMS to address the etiological factors have significantly improved the outcome of endodontic surgery. Indeed, outcome studies published about a decade ago have shown significantly higher success rates compared to those published more than two decades earlier [2, 60–68, 70–91]. Interestingly, outcome for nonsurgical root canal treatment has not been changed in this same time period [7–11]. As discussed above, the great technical differences that exist between TES and EMS to address intraradicular infection translate into great outcome differences.
There exists a variation in results of systematic reviews in regard to endodontic surgery. Torabinejad et al. [97] showed that the pooled success rate was 73.8 % with the weighted success rate 75.0 % based on 6,647 teeth from 26 studies. A recent systematic review by Tsesis et al. [59], however, showed that EMS had a pooled success rate of 89 % based on 1,576 teeth from 18 studies. Another systematic review by Tsesis et al. [58] reported that the pooled success rate of EMS was 91.6 % based on 880 teeth from 11 studies. This significant difference in success rates is attributed to the selection criteria of individual systematic reviews. Torabinejad et al. [97] did not distinguish EMS from TES in their criteria. Therefore, the pooled success rate in their systematic review [97] was inevitably lower than those in the systematic reviews of Tsesis et al. [58, 59] that included EMS only.
Naturally, there are fewer EMS outcome studies with long-term follow-ups than TES outcomes studies with long-term follow-ups. Therefore, when long-term success rates of endodontic surgery are reported, TES has more statistical weight on the long-term success rates. Indeed, Torabinejad et al. [97] showed that the pooled success rate was 77.8 % at 2–4-year follow-ups, which dropped to 71.8 % at 4–6-year follow-ups and further deteriorated to 62.9 % at more than 6-year follow-ups. Although limited in number, the EMS outcome studies with long-term follow-ups demonstrated that initial success rates remained high and fairly constant at +90 % over time [2, 83–91]. In contrast, the success rate of TES was initially ~69 % and further declined to ~56 % in long-term follow-ups [60–82]. This noticeable variation was due to the limitations in addressing the intraradicular etiologies in TES. Interestingly, TES still had more than 50 % overall long-term success rates, perhaps due to its ability to eliminate the extraradicular factors.
Prognostic Factors Affecting Outcome Variations
There are overt and hidden heterogeneities between outcome studies in the selection criteria and study designs that potentially affect the outcome. Therefore, one should understand the potential prognostic factors that influence the outcomes and, more importantly, how to assess the individual studies without bias. It is thought that many prognostic factors significantly influence the outcomes. These factors must be reevaluated from the perspective of modern surgical techniques in order to evaluate the outcome variations in current endodontic surgery.
Does Resurgery Have a Poorer Outcome than First-Time Surgery?
Peterson and Gutman [98] reported in their systematic review that only 35.7 % of 350 patients healed successfully after resurgery with 26.3 % uncertain and 38 % failed. This finding is consistent with Gagliani et al. [99] who showed that teeth that underwent resurgery had a poorer outcome compared to that of initial surgery, although this prospective study reported 76 % success rate (59 % complete healing and 17 % incomplete healing). Gagliani et al. [99] used a modern surgical technique including ultrasonic tips, EBA as the root-end filling material, and 4.5× loupes to aid visibility. Another prospective clinical study by Song et al. [100] showed a 92.9 % success rate after endodontic resurgery based on 42 patients with a 77.8 % recall rate. Song et al. [100] used ultrasonic tips and MTA and EBA as root-end filling materials with a surgical operating microscope. The success rate in this study is equivalent to those of first-time EMS and is thought to be due to the ability of EMS to address the causes of posttreatment endodontic disease. Therefore, resurgery may not be considered to affect the outcome negatively if microsurgical techniques are used.
Does High-Power Magnification Affect the Outcome of Endodontic Surgery?
Higher magnification aids in identifying the etiology of persistent apical periodontitis and provide a more precise control of surgical procedures. Surgical operating microscopes and endoscopes are considered to be state-of-the-art magnification tools for endodontic surgery. Any less magnification and illumination is thought to be associated with the lower success rates in TES compared to EMS. However, it should be noted that in order to investigate the effect of magnification on the outcome, magnification should be the only variable to compare, while the other variables such as surgical techniques and materials are controlled. Del Fabbro and Taschieri [101] reported that there was no significant difference in success rates among loupes, endoscopes, and microscopes. Setzer et al. [102] performed a meta-analysis based on 14 outcome studies, which applied the same ultrasonic root-end preparation and root-end filling with biocompatible materials but different magnification tools, and showed that endodontic surgery with the endoscope or the microscope had a statistically greater success rate than those with insufficient magnification. Interestingly, this systematic review showed that no significant difference was observed for anteriors and premolars, but there was a significant difference for molar surgery [102]. This finding is perhaps due to the low statistical power during the subgroup analysis. However, it may be also due to the easier accessibility and less complex root-end intricacies in anteriors and premolars. A recent meta-analysis by Tsesis et al. [59] showed that significantly higher positive outcomes were found when endoscopes or microscopes were used as a magnification tool compared to when loupes were used. This result is consistent with von Arx et al. [103], who reported that teeth that underwent endodontic surgery with the use of an endoscope had a higher success rate than teeth without the use of an endoscope. Therefore, higher magnification using the endoscope and microscope may be considered a prognostic factor that affects the outcome of endodontic surgery positively.
Does the Type of Root-End Filling Materials Matter?
The limited root canal disinfection can be achieved in endodontic surgery by root-end resection and root-end preparation. Therefore, remaining microorganisms in the root canal system may cause a recurrent apical pathosis if the apical seal is incomplete. In order to have an adequate apical seal, proper root-end filling materials should be used. MTA and EBA appear to have a better sealing ability than amalgam and other filling materials such as Cavit and zinc oxide eugenol [92]. In addition, the depth of root-end filling is an important factor for an adequate apical seal and should be at least 3–4 mm when MTA is used as a root-end filling material [104, 105]. MTA was reported to also have better biocompatibility compared to EBA and amalgam [96]. These results from preclinical studies give us considerable insights into selecting root-end filling materials for endodontic surgery, but we do need clinical outcome studies to validate whether superior sealing ability and biocompatibility of MTA can translate into higher success rates compared with other materials. In a systematic review by von Arx et al. [103], a significantly higher estimated healed rate was found in studies using MTA (91.4 %) as compared to amalgam (57.9 %) and glass ionomer cement (51.2 %). Notably, no significant differences in estimated healed rates were found among MTA, EBA (69.8 %), and IRM (71.6 %) [103]. A meta-analysis by Tsesis et al. [59], however, showed that use of MTA was associated with significantly higher success rates than the use of IRM or EBA, although MTA did not significantly differ from EBA in success rates when studies with low risk of bias were selected and analyzed. A recent retrospective study by Song et al. [106] demonstrated a significant difference between MTA and IRM, but no difference between MTA and EBA. In contrast, two randomized controlled study showed no significant difference between MTA and IRM [85, 107]. Clearly, there is no doubt as to the better clinical outcomes with MTA compared to other materials, although MTA, EBA, and IRM are considered clinically acceptable root-end filling materials. Therefore, the material selection for root-end filling is considered to affect the outcome significantly.
Does Periodontal Involvement Worsen the Outcome?
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


