Use of the microbiology laboratory
Publisher Summary
Clinical oral microbiology is a new discipline and involves the study of specimens taken from the patients who are suspected of having oral infections. The end result is a report that should assist the clinician in reaching a definitive diagnosis and help in deciding on therapy. Clinicians should be conversant with the appropriate techniques of specimen taking and comprehend at least the principles of microbiological analysis. This chapter discusses the ways and means by which clinicians could make the optimal use of the laboratory. It describes specific laboratory procedures, such as sensitivity testing. The resources of a microbiology laboratory can be efficiently utilized by sending appropriate specimens that are properly collected. Specimens should be as fresh as possible for optimal isolation of the pathogens. The specimens should be transported as soon as possible to the laboratory in an appropriate transport medium. In the absence of a transport medium, dehydration of the specimen and exposure of the organisms to aerobic conditions occur, with the resultant death or reduction in their numbers.
Requesting a microbiology report
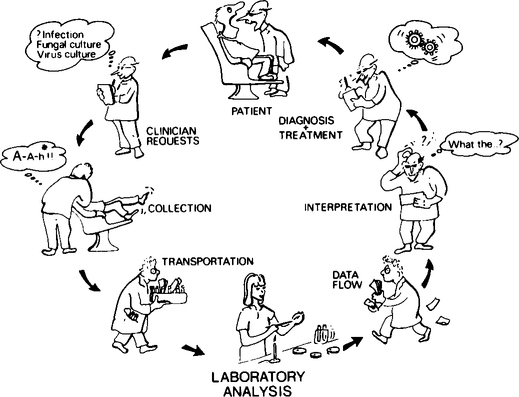
A spectrum of decisions and actions by a number of individuals are involved from the time the clinician decides to take a microbiological sample until he receives the laboratory report. This spectrum of events can be conveniently divided into three categories: (1) ordering, collecting and transportation of specimens; (2) microbiological laboratory processing and reporting; and (3) interpretation and use of information (Figure 13.1).
Laboratory analysis
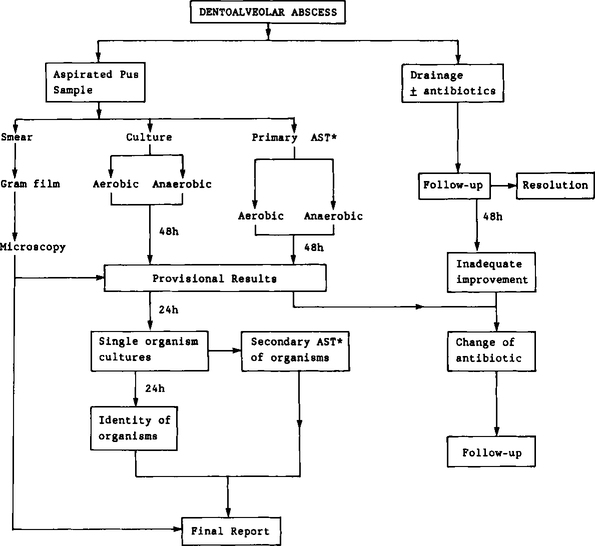
In order to give the reader an insight into the laboratory analysis of specimens, a brief account of the analytical pathway of a pus specimen is given below and is shown in Figure 13.2.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses