• Figure 8-1 Moistened gauze surrounding surgical site to absorb stray laser energy and protect surrounding structures before removal of a tongue lesion.
The most frequent adverse sequelae resulting from the use of lasers are caused by laser energy emitted beyond the working area and striking the surrounding soft tissues. Such misdirection typically occurs when a specimen is transected, because the laser energy passing beyond the margin may redirect off a reflective metal surface of an instrument in or near the oral cavity (e.g., a mirror or other flat retractor). These events usually result in minimal or no injury, but the patient’s initial response to the stimulus may be interpreted as pain. This problem can easily be avoided by obstructing the distant tissues with moist gauze or a wet tongue blade and by using matte-finished, nonreflective instrumentation.3
Wavelength-specific eyewear is mandatory, protecting the user from misdirected or reflected laser energy. During laser operation, high-speed suction or evacuator devices should be used and high-filtration masks should be worn to prevent illness from inhalation of the plume released at the site of energy–tissue interaction. To prevent ignition with flammable gases, nitrous oxide and oxygen should ideally be temporarily discontinued during laser use, to prevent serious harm to the patient. Because CO2 laser energy is well absorbed by hydroxyapatite, a major component of tooth enamel, significant amounts of laser energy absorbed by the teeth can cause etching and pitting, weakening enamel37 and increasing pulpal temperature.38 When in proximity to the tip during a CO2 laser procedure, the teeth can be protected with moistened gauze or a fabricated tooth guard to absorb errant energy from the laser beam (Figure 8-1).
In general dentistry practice, the following three fundamental photothermal techniques for CO2 laser applications can be used to perform various intraoral procedures:
• Incision/excision surgery (Figure 8-2)
• Ablation/vaporization procedures (Figure 8-3)
• Hemostasis/coagulation techniques (Figure 8-4)
All minor office-based oral surgical procedures are based on these three techniques. The first procedure discussed here is biopsy; however, the incisional techniques used to perform biopsies are applicable to virtually every intraoral procedure.
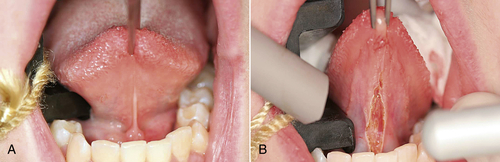
• Figure 8-2 Example of incision/excision procedure: lingual frenectomy. A, Preoperative view; B, immediate postoperative view.
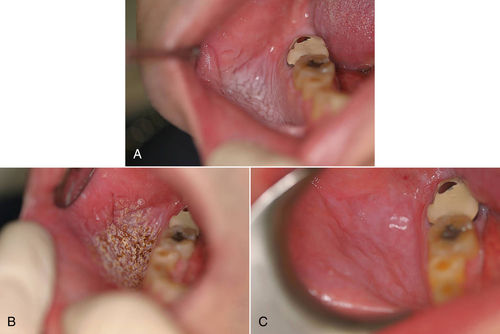
• Figure 8-3 Example of ablation/vaporization of a previously biopsied leukoplakic lesion. A, Immediate preoperative view; B, intraoperative view; C, 12-day postoperative view.
It is important to recognize that one or more techniques may be necessary for any given clinical scenario, depending on three surgeon-controlled laser parameters: energy, time, and spot size.3 These parameters equate at the focal point of the laser energy emitted from any given laser handpiece. Altering the distance of the handpiece from the tissue serves to focus and defocus the laser beam’s focal point, thereby altering the effect of the laser on the target tissue. A laser in focus will excise and incise with the most efficiency. When the laser is out of focus, depth of effect will be less, but with a wider field of effect for ablation and coagulation.
Incision/Excision Techniques and Procedures
With focused mode, the focal point of the laser beam is at such a distance as to exactly focus on the tissue to be affected (but not in contact with the handpiece), maximizing the power per unit to a pinpointed area. Using the CO2 laser in focused mode allows for increased depth, yet the laser can produce an incision similar to that made by a scalpel, essentially functioning as a “light” scalpel. The characteristics of the CO2 laser make it ideal for most intraoral procedures traditionally performed with a scalpel, such as incision and excision, lesion removal, and flap elevation.3,7,39
Biopsy Procedure
Every patient requires a screening for cancer, which is accomplished by performing a thorough oral, head, and neck examination. Oral examinations can be made more efficient by inspecting high-risk sites, where 90% of oral squamous cell cancers arise: floor of the mouth, ventrolateral aspect of the tongue, and the soft palate complex.40 Many screening and detection aids can be used as adjunctive tools to identify precancerous and cancerous tissues early and before they become visible to the naked eye, expediting diagnosis and improving the patient’s prognosis. Oh and Laskin41 noted visual accentuation of some lesions when patients used acetic acid rinse, but with no significant improvement in detection. Also, examination with chemiluminescent systems can produce reflections that make visualization more difficult.
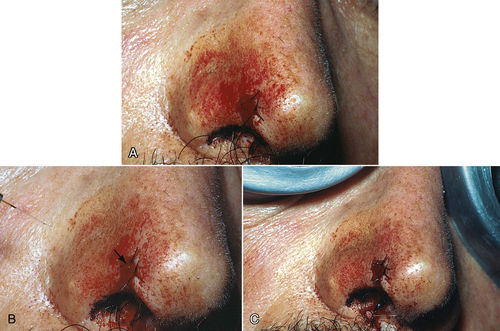
• Figure 8-4 Example of hemostasis/coagulation procedure in a patient on “blood thinner” medication. A, Preoperative view showing bleeding from a sutured excision biopsy of lesion on the nose. B, Intraoperative view. Note Nd:YAG laser fiber on the left side and the aiming beam shining on bleeding site (arrow). C, Immediate postoperative view after use of laser to coagulate the bleeding site. (Courtesy Dr. Robert Convissar.)
When cellular changes are detected, the limitation of all adjunctive techniques is that a surgical biopsy procedure remains necessary to obtain a diagnosis.42–44 Biopsy is the process of removing a sample of tissue from the patient for diagnostic examination. This procedure is required to determine the underlying process within the tissues that is causing changes in their clinical appearance. The pathologic diagnosis obtained ranges from “normal” to an inflammatory process to a benign or malignant neoplasm. Ultimately, an accurate diagnosis guides the clinician in determining the necessary treatment. The five intraoral biopsy techniques are aspiration biopsy, cytologic biopsy, brush biopsy, excision biopsy, and incision biopsy.
The brush biopsy technique has improved the sensitivity (92.3%) and specificity (94.3%) for detection of oral squamous cell carcinoma or dysplasia when tested on visually identified lesions.45–47 Of note, brush biopsies in particular should be used only as a screening tool, and atypical cell identification or a positive result from such biopsies will necessitate an additional step: implementing a surgical procedure to confirm a diagnosis.48 The main problem with brush biopsy is inadequate depth of the specimen resulting from operator error.
Formulating a definitive diagnosis generally requires the acquisition of a tissue specimen histologically representative of the lesion, using one of the two surgical biopsy techniques. Until recently, most surgical biopsies have been performed with “cold steel”—the scalpel. The disadvantage of this approach is that patients frequently experience significant intraoperative and postoperative sequelae.
The preferred modality for the intraoral surgical biopsy is the laser, for several reasons. The laser’s hemostatic nature creates a blood-free surgical field compared with the scalpel. This is critical with vascular lesions or in treating patients who tend to bleed excessively; the laser minimizes blood loss. Other advantages of using a laser are reduced surgery time (superior precision of incision) and unparalleled visualization of the surgical site (lack of blood in surgical field). Decreasing the duration of surgery reduces tissue manipulation and potential wound contamination. The laser’s thermal effect produces minimal lateral thermal necrosis but provokes enough response to attain a bactericidal effect. Patients are comfortable during the procedure with minimal local anesthesia. After laser surgery, patients quickly return to their daily routine, experiencing no bleeding or swelling. Discomfort usually is minimal and generally can be treated with OTC nonnarcotic analgesics such as ibuprofen. Surgical biopsies with a laser often can be performed on the patient’s initial visit, accelerating the diagnostic process and expediting treatment.
Incision Technique
The location and size of the lesion dictate whether incision or excision surgical biopsy technique should be used. The incision technique removes only a representative portion or portions of a lesion as well as adjacent normal tissue. Superficial lesions described as being leukoplakic and erythroplakic, with histologic features consistent with hyperkeratosis, lichen planus, leukoedema, epithelial dysplasia, carcinoma in situ, or squamous cell carcinoma, usually are treated with this technique. Frequently, multiple incision biopsies at several locations are required to obtain tissue for microscopic examination when oral cancer is suspected.
The incision biopsy specimen obtained using a scalpel typically is elliptical in shape and should be of sufficient depth and width for obtaining a deep tissue margin, taking into account the infiltrating properties of a carcinoma. When incising the lesion with the laser, it is imperative to generate an adequate specimen. Consideration must be given to the local extent of both healthy and diseased tissue, with careful attention to the possibility of lateral thermal necrosis. The biopsy specimen should extend down into the submucosa to permit determination of depth of invasion, to maximize the possibility of removing the lesion, and to decrease the likelihood of seeding cancerous cells into the surrounding cells.
Excision Technique
Excision technique requires the removal of the entire lesion with at least 2 to 3 mm of peripheral margin (Figure 8-5 A–C). This technique is preferred for oral lesions 1 cm or less and for minor, solid, and exophytic lesions. Localized discrete lesions such as fibroma, papilloma, mucocele, and pyogenic granuloma are most often excised with a laser.
When surgeons perform their first laser biopsy, they should take slightly wider margins than they would take with a blade, to decrease the possibility of thermal necrosis (a beginner’s mistake in biopsy technique) at the incision margins.39
When an excision biopsy is confirmed to contain a positive margin of disease, this procedure is classified as an “incision” biopsy. Additional treatment is then required to eradicate the disease.
Documentation
Digital photography is invaluable in dental practice today. A clinical photograph should be captured before administering anesthetic to preclude capturing any distortion in the involved tissues. It is vital to document all aspects of a patient’s treatment, including presurgical and postsurgical photographs, surgical margins, any surgical defects, and the biopsy specimen (see Figure 8-5 E). A specimen submitted for review should contain any pertinent medical and clinical history, with photographs attached to assist the pathologist in formulating a diagnosis. If a diagnosis is questionable or a malignancy is suspected, it is best to discuss concerns with the pathologist and always perform another biopsy as appropriate.
Anesthesia
Local anesthesia should be used to ensure optimal comfort for the patient. If local anesthetic is placed directly on the lesion or planned incision margin, the fluid content in the tissue from the injection may lead to both altered tissue removal and inconsistent cutting secondary to absorptive properties of the laser energy. Performing neural blocks, deep infiltration, or infiltration at least 1 cm away from the lesion is ideal and will minimize distortion to the surgical area.
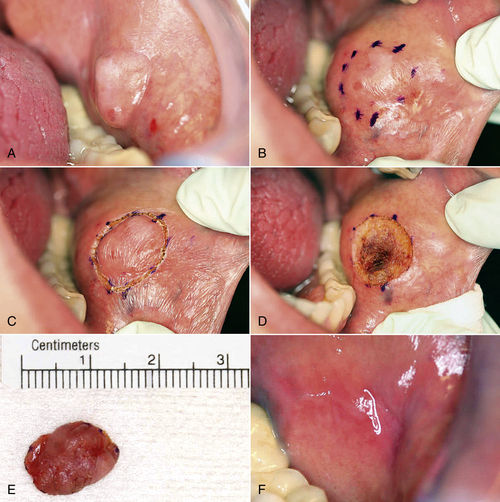
• Figure 8-5 Excision biopsy technique. A, View of the presenting lesion shows surrounding anatomy. B and C, Outlining the planned incision with a marking pen or with the laser at a lower power setting allows for greater control with incision placement. D, Ideal hemostasis within surgical defect. E, Photographic documentation of specimen. F, One-week postoperative view.
Other Incision/Excision Procedures
Laser incision and excision techniques also are applicable to other intraoral procedures. These techniques are lesion-independent; any lesion or tissue requiring incision or excision is treated using the same basic method previously outlined.7 Lasers have proved to be successful in correcting many oral soft tissue anomalies.49,50
The parameters for incision and excision procedures vary by laser, as well as by type of tissue being treated and the experience of the clinician. Adherence to “cookbook” parameters occasionally recommended in the literature should be avoided; these may not be consistent with the expected tissue effect in a particular case (Case Studies 8-1, 8-2, and 8-3).3
Ablation/Vaporization Techniques and Procedures
The CO2 laser is unique in its ability to function as a “light scalpel” and a photothermal vaporizer. Tissue ablation/vaporization is a technique performed with lasers in defocused mode and achieved by moving the laser away from the tissue beyond the focal point, causing an increase in spot size that directly decreases power density and depth of the cut. The absorbed energy vaporizes the tissue in a controlled, predictable manner. Cryosurgery and chemical peeling are similar but unpredictable because of the inability to achieve a constant depth and the difficulty of applying these modalities intraorally. The noncontact nature of the CO2 laser makes this wavelength the best of those available for ablation, although the use of wide optical fibers (e.g., 800 μm) may allow other lasers to be used as well.
Laser vaporization is the safest, fastest, and most predictable surgical modality available today. The ablation technique often is used to treat discrete intraoral lesions, benign and premalignant surface lesions, and inflammatory disease, as well as for contouring gingival tissues for functional and esthetic purposes. Other common applications include the management of epithelial hyperkeratosis, hyperplasia, dysplasia, lichen planus, and nicotine stomatitis.
Vaporization Technique
Vaporization of a lesion precludes a histologic diagnosis. Accordingly, vaporization should be performed only in areas for which biopsy specimens have already been obtained or when a reasonable presumed diagnosis has been made.3
Vaporization is ideal for removal of large surface lesions confined to the epithelium, located in areas such as the floor of the mouth, where incisions are likely to compromise the underlying anatomy. In such cases, traditional biopsy techniques using a scalpel would be considered aggressive because they eradicate too much tissue and may cause bleeding, scarring, and injury to adjacent structures. With most CO2 lasers, each pass during tissue ablation penetrates from a few hundred micrometers down to 1 to 2 mm. By selectively removing each cellular layer, laser ablation can be completed in a conservative fashion, with minimal insult to the underlying tissue and structures. After laser ablation, tissue elasticity remains resilient, scarring is reduced, and fundamental function is preserved.22,23
Lesion Treatment
Superficial lesions that demonstrate leukoplakia, erythroplakia, or a combination are more likely to undergo malignant transformation. Affected patients have a 50- to 60-fold greater risk of developing oral cancer.7 Laser ablation of these lesions is considered controversial. Interventional laser excision or ablation of precancerous oral epithelial lesions offers unique advantages, including elimination of diseased tissue, control of blood loss, favorable patient acceptance, low morbidity with reduced complications, and successful healing.51
Studies have demonstrated that laser ablation with regular follow-up evaluations is effective in controlling dysplastic lesions of all grades. The recurrence rates for premalignant lesions are not significantly different for scalpel excision and for laser vaporization. Vedtofte et al.52 reported a 20% recurrence rate over 4 years in patients who underwent scalpel excision. Horch et al.53 reported a 22% recurrence rate over 37 months in patients who underwent laser vaporization. Thompson and Wylie54 reviewed data for 57 consecutive laser-treated patients presenting over a 4-year period with histologically confirmed dysplastic lesions. Over 44 months, findings showed 76% of patients remained disease-free, comparable to the 80% success rate in patients treated with surgical excision.
Laser vaporization is an effective, nonmorbid, inexpensive, quick, and relatively painless method of managing premalignant lesions. Many clinicians believe that the hemostatic effect of the laser results in decreased tendency for hematogeneous or lymphatic seeding of the malignant cells.55,56 The low morbidity and minimal pain generally associated with laser ablation make it a valuable tool in the management of premalignant mucosal lesions.
Ablation Technique
Regardless of the type of ablative laser used to perform this procedure, the ablation technique is accomplished using a defocused mode, which increases the spot size and decreases the power density and depth of cut. The depth of ablation is increased by using a higher power setting and decreased by moving the handpiece faster or by increasing the spot size. The size and depth of the lesion help determine the power setting and spot size for treatment.
As with excision biopsy, the clinician should begin by outlining the circumferential margins. The outlined margins serve as surgical boundaries, extending 0.5 cm beyond the identifiable lesion. Ablation of the lesion is accomplished through a continuous series of connecting parallel Us within the delineated margins, taking care not to leave any lesion present. This method ensures an evenly ablated surface. Overlapping ablative passes can result in greater lateral thermal damage and increased depth. Ablated tissues easily become dehydrated; therefore, with retreatment of a previously irradiated area, increased lateral thermal damage may occur. To minimize the likelihood of a poor outcome, the tissues should be rehydrated with water spray, and any surface carbonization should be gently wiped away with moist gauze between passes.3 To achieve deeper penetration, additional passes may be performed perpendicular to the initial ablative pattern, to ensure complete coverage of the lesion (Figure 8-9 and Case Studies 8-4 to 8-6 with Figure 8-7).
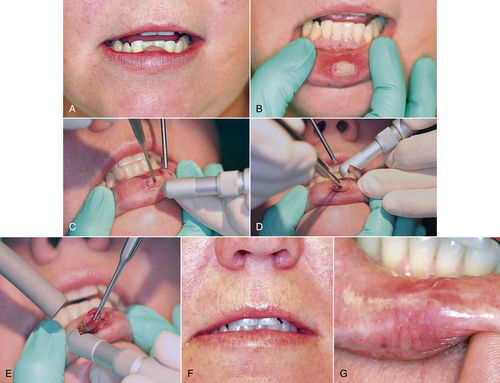
• Figure 8-6 A and B, Pyogenic granuloma is a clinically recognizable lesion and usually manifests as an inflammatory soft, red, raised mass that often bleeds when traumatized. C and D, Incision is performed after delineation of the surgical margins at lesion’s periphery. E, Complete excision at base of lesion. F and G, At 10 days, suture removal is completed. An early esthetic result is evident.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


