Appropriate treatment of implants is becoming increasingly important for the general dentist as the number of implants placed per year continues to increase. Early diagnosis of peri-implantitis is imperative; initiating the correct treatment protocol depends on a proper diagnosis. Several risk factors exist for the development of peri-implantitis, which can guide patient selection and treatment planning. Treatment of peri-implantitis should be tailored to the severity of the lesion (as outlined by the cumulative interceptive supportive treatment protocol), ranging from mechanical debridement to explantation. Several surgical and nonsurgical treatment alternatives exist. There is little consensus on superior treatment methods.
Key points
- •
Appropriate supportive treatment of implants is becoming increasingly important for the general dentist as the number of implants placed per year continues to increase.
- •
Early diagnosis of peri-implantitis is imperative, and initiating the correct treatment protocol depends on a proper diagnosis.
- •
Several risk factors exist for the development of peri-implantitis, which can guide patient selection and treatment planning.
- •
Treatment of peri-implantitis should be tailored to the severity of the lesion (as outlined by the cumulative interceptive supportive treatment protocol), which ranges from mechanical debridement to explantation.
- •
Several surgical and nonsurgical treatment alternatives exist, and there is little consensus on superior treatment methods.
Introduction
Dental implants have revolutionized the treatment of tooth loss to the extent that they are now considered the standard of care in many circumstances. Although implants are now a very predictable treatment option, occasionally they fail for a variety of reasons. Peri-implantitis is a late complication of dental implants and is the primary process that leads to late failure. This article reviews definitions, risk factors, diagnosis, and therapy for peri-implantitis.
Since the first dental implants were placed by Brånemark in 1965, they have experienced enormous success and growth. The number of implants placed in the United States increased more than 10-fold from 1983 to 2002 and increased another 10-fold from 2000 to 2010. The number of dental implants placed in the United States per year is roughly 5 million, and this number is projected to sustain yearly growth at 12% to 15% for the next several years. With the ever-increasing number of implants, management of attendant complications (such as peri-implantitis) will become increasingly important for all clinicians involved in the patient’s care.
Introduction
Dental implants have revolutionized the treatment of tooth loss to the extent that they are now considered the standard of care in many circumstances. Although implants are now a very predictable treatment option, occasionally they fail for a variety of reasons. Peri-implantitis is a late complication of dental implants and is the primary process that leads to late failure. This article reviews definitions, risk factors, diagnosis, and therapy for peri-implantitis.
Since the first dental implants were placed by Brånemark in 1965, they have experienced enormous success and growth. The number of implants placed in the United States increased more than 10-fold from 1983 to 2002 and increased another 10-fold from 2000 to 2010. The number of dental implants placed in the United States per year is roughly 5 million, and this number is projected to sustain yearly growth at 12% to 15% for the next several years. With the ever-increasing number of implants, management of attendant complications (such as peri-implantitis) will become increasingly important for all clinicians involved in the patient’s care.
Definition of terms
To discuss peri-implantitis, it is important to briefly differentiate it from other forms of bone loss around implants. These other forms of bone loss include those that could be primarily biomechanical in nature (eg, implant fracture, occlusal overload, and marginal bone loss) as well as those that are primarily inflammatory in nature (eg, peri-implant mucositis and retrograde peri-implantitis). Although bone loss is a shared end result, there are distinctions of probable cause, probable course, and current treatments. The ongoing process of reaching consensus is important, because it will affect reporting of prevalence, as well as the inclusion of studies in systematic reviews and the use of data in meta-analyses.
Marginal Bone Loss
As originally described in 1986 by Albrektsson and colleagues, marginal bone loss allowed for 1.2 mm of bone loss the first year following abutment connection and 0.2 mm per year thereafter, later to be modified as 1.5 mm of bone loss in the first year followed by 0.2 mm per year thereafter. This bone loss occurs in the absence of clinical symptoms, and, as is the case with peri-implant mucositis and peri-implantitis, has multiple potential contributing factors.
Retrograde Peri-Implantitis
First described by McAllister and colleagues, retrograde peri-implantitis is a radiographically diagnosed, periapical, lucent lesion that is symptomatic and that develops shortly after implant placement. The coronal portion of the implant appears to have a normal relationship to bone. In some cases, a fistula may develop. It is likely related to microbiological conditions at the implant site and has been shown to be related to distance from site and time since endodontic therapy.
Peri-Implant Mucositis
Peri-implant mucositis is an inflammatory process around a functioning implant characterized by bleeding on probing (BOP), with depths of 4 mm or more. There is no indication of bone loss other than that which would normally be expected with marginal bone loss and it may or may not be accompanied by suppuration. It is considered to be reversible without procedural intervention, other than perhaps scaling and root planing. Although it is generally considered to be the precursor to peri-implantitis, it is not necessarily true that it will progress to peri-implantitis.
Peri-Implantitis
Similar to peri-implant mucositis, peri-implantitis is inflammatory in nature, but is considered distinct in that it is not considered to be reversible without surgical intervention and has bone loss around a functioning implant beyond that which would be expected by normal bone remodeling. The bone loss threshold used to make that distinction lacks consensus, as reflected by the fact that prevalence can vary as much as 47% to 11%, depending on where the threshold is set ( Table 1 ).
| BOP ± Suppuration | Probing Depth ≥4 mm | Radiographic Bone Loss | |
|---|---|---|---|
| Peri-implant mucositis | + | + | − |
| Peri-implantitis | + | + | + |
The earlier in its progression that peri-implant disease can be identified, the better the prognosis for the implant in question, thus underscoring the importance of periodontal probing.
Risk factors
A risk factor is any attribute, characteristic, or exposure of an individual that increases the likelihood of developing a disease or injury. Elucidating risk factors and the conditions predisposing to peri-implantitis gives insight into the pathophysiology of the disease and will allow for the eventual development of concepts of disease prevention and logical treatment strategies.
Numerous contributing factors for peri-implantitis have been considered. A limited number have been selected for discussion. Nevertheless, arriving at a clear and evident cause, or causes, remains elusive.
Diabetes
Although periodontal disease is considered to be a complication of diabetes, the relationship between diabetes and peri-implantitis remains unclear.
In rodent studies, sustained hyperglycemia as a result of type 1 or type 2 diabetes mellitus will lead to deleterious changes in the periodontium, including impairment of host defense against pathogens, prolonged inflammatory response, microvascular alterations, impairment of new bone formation and repair, and impaired wound healing. As similarly explained for the human model, these changes have the potential to result in increased susceptibility of diabetics to periodontitis, which can be explained by multiple mechanisms in both models. Hyperglycemia is also known to delay wound healing, supporting the recommendation for allowing extended osseointegration periods for diabetics.
Given the similarities between periodontitis and peri-implantitis and the deleterious effect that diabetes has been noted to have on soft tissue, periodontal membranes, and alveolar bone, it would be reasonable that studies would demonstrate a relationship between diabetes and peri-implantitis. Nevertheless, despite these similarities, systematic reviews of studies attempting to demonstrate a possible relationship do not show a clear linkage between them; this could be due to an inadequate number of studies actually assessing glycemic control as part of the study, because chronic hyperglycemia is the underlying issue, as opposed to a diagnosis of diabetes, which can be well-controlled. Although the intricacies of a possible relationship may eventually become apparent, (ie, quality of normoglycemic control, other comorbidities), it remains to be seen if future studies and systematic reviews will reconcile this apparent lack of correlation.
Occlusal Overload
Occlusal overload is another risk factor influenced by prosthetic design; however, it is very difficult to define and quantify clinically over the lifetime of an implant. Thus, it is a very difficult risk factor to study (especially across different studies that all may have different definitions and methods of quantifying occlusal overload). However, a systematic review by Fu and colleagues concluded that occlusal overloading was associated with peri-implant marginal bone loss because this type of bone loss is likely caused directly by microtrauma and because occlusal load is concentrated at the marginal bone, this bone loss is likely to occur via a different pathophysiologic process than other forms of peri-implantitis. Again, more studies with more uniform criteria for occlusal loading are necessary.
Genetic Factors
The host response to bacterial insult is extremely complex and influenced by many host genes. Pathogenic species stimulate proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, which coordinate many aspects of the local inflammatory response. TNF-α subsequently induces more adhesion molecules, proinflammatory cytokines, and chemokines as well as induces osteoclast function resulting in bone loss. Peri-implant crevicular fluid TNF-α may be an indicator for peri-implant inflammation and may precede indicators such as BOP increased probing depth (PD), providing for an earlier diagnosis.
There is potential for genetic alterations of any of the above factors to influence peri-implantitis. IL-1 is a group of 11 cytokines that are involved in the regulation of immune and inflammatory responses. There is conflicting evidence regarding IL-1 gene polymorphism and its link to peri-implantitis. Several studies suggest a link between the two, for example, as shown with polymorphism of the Interleukin-1 receptor antagonist gene (IL-1RN). However, a 2010 systematic review found no consensus among the studies reviewed. Further studies are necessary to determine which genetic markers, if any, can be used to predict patient susceptibility to peri-implantitis.
Residual Cement
Restoration design influences several of the known peri-implantitis risk factors, including plaque control, occlusal overload, and also the likelihood of residual cement. Unfortunately, the vast majority of cements used for implant restorations are radiolucent; thus, clinicians cannot reply on radiographic confirmation of clean margins. Stock abutments that do not follow the gingival contours make interproximal cement removal difficult or impossible, and accessible margins can become esthetic issues. It is unclear whether the progression to peri-implantitis results from the roughness of the cement (which alone can cause inflammation) or from providing a suitable environment for bacterial attachment. A study by Wilson evaluated 42 implants with signs of peri-implantitis and compared them to 20 other asymptomatic implants in the same patients. Using endoscopic visualization of the margins, he found that 0% of the asymptomatic implants had extruded cement, whereas 81% of the symptomatic implants had extruded cement. Of these with extruded cement, 76% had resolution of symptoms with removal of cement (either as a closed procedure with endoscopy, or rarely, requiring flap reflection to achieve complete removal); this underscores the importance of techniques to avoid cement extrusion at the time of cementation and suggests screw-retained restorations as the ideal restoration if possible.
Smoking
The numerous deleterious effects of cigarette smoking on health are well-documented and appear in most cases of smoking-related pathoses to share a common pathway of alterations in the immune-inflammatory system.
The literature is replete with reports from various disciplines documenting the negative effects of smoking on multiple organs and body systems. For example, it has been shown that osteotomies in smokers require a 42% increase in time over nonsmokers to achieve radiographic bone consolidation. There is a strong association between smoking and dermatologic conditions, including skin wound healing, wrinkling, and the effects of premature aging as well as other conditions. Smokers showing impaired wound healing after breast reduction surgery also have higher levels of urine cotinine both preoperatively and postoperatively than do nonsmokers.
In order for any wound to heal properly, the 4 stages of healing (hemostasis, inflammation, proliferation, and remodeling) need to occur in the proper sequence and be of proper duration. Although smoking has been shown to attenuate the normal inflammatory healing response by a reduction in inflammatory chemotactic responsiveness, migratory function, and oxidative bactericidal mechanisms, there is also evidence that in both diabetes and periodontal disease, a condition of hyperinflammation is established. These immunomodulatory effects of smoking, either pro-inflammatory or suppressive, can lead to an altered inflammatory response, including chronic inflammation, at mucosal surfaces.
In recognizing the documented effects that smoking has on healing tissues, it would be reasonable to assume that cigarette smoking would have similar negative effects in the oral cavity as it has with periodontal disease.
Combining this information with the findings that smoking cessation mitigates against the progression and incidence of periodontitis, a compelling argument can be made for the negative effects of smoking on periodontal disease. However, recent studies have suggested that smoking has been shown to be a weak predictor of periodontitis and that it may take up to 30 years or more of smoking before its impact becomes clinically significant.
The attempt to delineate the relationship of smoking to peri-implantitis shows that it runs a similar course as that with periodontitis. There are ample studies to show that there is a negative effect of smoking on implants, including contributing to peri-implantitis.
Nevertheless, although these studies arrive at the logical conclusion linking smoking to peri-implantitis, there are other reports that confound this assumption. A recent systematic review and meta-analysis by Sgolastra and colleagues brings that association into question. This study states that while an implant-based meta-analysis reveals a higher risk of peri-implantitis in smokers compared with nonsmokers, the patient-based meta-analysis did not reveal any significant differences in risk of peri-implantitis. This finding could be due to a small number of studies fulfilling the inclusion criteria, and further studies are suggested to prove or disprove the link. Prior studies help support this conclusion.
Just as with diabetes, a clear linkage of cigarette smoking to peri-implantitis is problematic both at the level of individual studies and at the level of systematic reviews and meta-analyses.
Periodontal Disease
Intuitively, the patient population with dental implants would have a higher than average history of periodontal disease, because periodontal disease is the most common reason for teeth to be extracted and subsequently replaced with implants. Systematic reviews have shown that a history of periodontitis increases the likelihood that an implant will have peri-implantitis. Interestingly, these systematic reviews suggest that this does not appear to negatively influence the implant survival rate; however, several recent long-term prospective cohort studies indicate decreased implant survival when implants are placed to replace teeth lost due to periodontal lesions compared with other reasons.
Another important consideration is how the existing periodontal disease affects the “quality” of the patient’s biofilm. Healthy peri-implant biofilms consist of low total bacterial loads that are primarily gram-positive cocci (similar to natural teeth). The progression to peri-implantitis follows the same general course as the progression to periodontitis, resulting in increased total bacterial load and, specifically, increased proportions of Aggregatibacter actinomycetemcomitans , Fusobacterium species, Prevotella intermedia , Porphyromonas gingivalis , mobile organisms, and spirochetes (ie, Treponema denticola ). Specifically, A actinomycetemcomitans and P gingivalis , which can be found in healthy peri-implant biofilms in small numbers, have been suggested to be the predominant pathogens implicated in peri-implant destruction.
A major difference between implants and teeth (in a partially edentulous case) is that with implants, one is introducing a sterile object into an environment of widely variable existing microbiota, so the most important question to answer is whether the existing microbiota influences the progression to peri-implantitis. Quirynen and colleagues showed that colonization of an implant occurs within 2 weeks of healing abutment placement, and that this flora was nearly identical to the adjacent teeth (same quadrant). Another indirect finding that would suggest the existing microbiota influences the likelihood of peri-implantitis is that implants in partially edentulous environments harbor more pathogenic peri-implant microflora than do their counterparts in fully edentulous environments. Also, higher plaque levels in fully edentulous sulci do not lead to impaired peri-implant conditions when compared with partially edentulous sulci. Further studies are necessary to determine if the presence of specific microbiological characteristics before implant placement can predict future peri-implantitis, or whether it is a concomitant finding that peri-implant microflora closely resembles that of periodontal lesions through its own separate pathway.
Poor Plaque Control
There are many factors that can lead to poor plaque control, although the one most unique to implant restorations (compared with natural teeth) is prosthetic design. The design of the prosthesis (whether single unit or full arch) has the potential to create uncleansable situations, and this must be considered when treatment planning (often there is a compromise between esthetics and cleansability). The surgical implant position must also be in harmony with the planned prosthesis to attain planned cleansability. Prosthesis design also has the potential to preclude clinical evaluation with periodontal probing, which may delay diagnosis of peri-implant mucositis or early peri-implantitis. Depending on the “quality” of the patient’s biofilm (discussed above), plaque control can potentially be more or less crucial for success in different patients.
Therapeutic rationale and treatment approaches
In the broadest sense, the goal of treatment of peri-implant diseases should be at a minimum to restore the implant and patient to a state of acceptable health and function. Although all the variables of success have yet to be agreed on, there are logical and generally accepted quantifiable clinical parameters that can be used as monitors. These clinical parameters include reduction of periodontal PD, improvement in clinical attachment level, reduction of BOP, and radiographic bone fill. It may or may not be possible, or even necessary, to re-establish osseointegration ; it may only be possible to fill the osseous defect or to simply arrest the disease. As more cases of peri-implant disease are treated by the various modalities and protocols, relapse and the need for re-treatment may become considerations as well. Also, given the large constellation of possible contributing factors, successful treatment may take on different meanings for various factors unique to the patient, the lesion, and the design of the implant. As further progress is made, the use of biomarkers may go hand in hand with clinical parameters, allowing for better predictability and selection of appropriate treatment rationale.
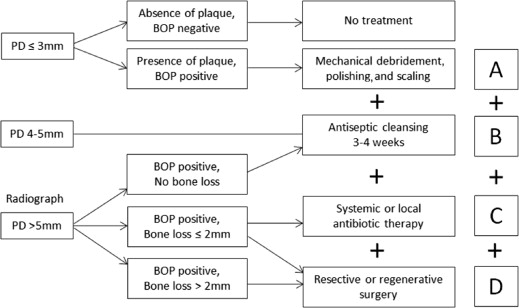
The cumulative interceptive supportive treatment (CIST) protocol was designed to tailor treatment of peri-implant lesions as related to easily measured clinical parameters and has been generally accepted since its introduction in 2000. It provides a progressive algorithm of treatment recommendations depending on the severity of the lesion. This protocol is briefly reviewed as well as alternatives to each aspect. It is important to remember that these protocols are additive (ie, the success of protocol C depends on the successful implementation of protocols A + B, and so on) ( Fig 1 ).