Introduction
Pain is a major concern of patients before orthodontic treatment. Currently, the most frequently recommended treatments for pain after archwire placement or appliance adjustment are over-the-counter (OTC) analgesics. Although the overuse of OTC medications and their potential side effects are concerns, particularly for children, no study to date has investigated a nonpharmacologic option for pain management as an alternative for these analgesics.
Methods
A parallel 2-group stratified block randomized clinical trial was designed to assess the pain response of adolescents during the first week after initial archwire placement. The subjects were randomly assigned to 1 of 2 pain management groups: bite wafer (BW) or OTC analgesics. Pain levels were reported on a numerical rating scale. The intensity and unpleasantness of the pain were also assessed. Data were collected at 8 times over a 7-day period. A general linear mixed model with heterogeneous compound symmetry covariance matrix was fitted separately for each outcome. Estimates from the mixed model were used to test a noninferiority hypothesis that the BW group, on average, was not inferior with respect to pain management to the OTC group.
Results
The patterns of pain level, intensity, and unpleasantness over time were similar for the 2 groups ( P >0.33). Pain management for the BW group as indicated by pain level, intensity, and unpleasantness was not inferior to that of the OTC group ( P >0.39).
Conclusions
In adolescents, the BW is a nonpharmacologic option for pain management after orthodontic procedures that is at least as effective as OTC analgesics.
Fear of pain and discomfort is a concern for many prospective orthodontic patients, and patients in treatment often describe the discomfort as pressure, tension, ache, or soreness of the teeth. The pain intensity usually increases gradually from 2 hours after application of orthodontic force to a peak at 24 hours, with resolution of the pain by the seventh day.
The most frequently recommended treatments for pain are over-the-counter analgesics (OTC), often a nonsteroidal anti-inflammatory drug (NSAID) that works by inhibiting prostaglandin synthesis. Previous studies assessing the efficacy of analgesics for pain management have focused on medications administered immediately before, or before and immediately after the orthodontic procedures. All of these studies reported that analgesics were effective for pain management.
The overuse and potential side effects of OTC analgesics have been raised as a concern, particularly for children. Because of these concerns, nonanalgesic pain management approaches such as chewing gum or chewing on a plastic wafer have been recommended to loosen the tightly grouped fibers around the nerves and blood vessels, restoring normal vascular and lymphatic circulation, thus preventing or relieving inflammation and edema. Although several studies have indicated that the rhythmic chewing of either Aspergum (Insight Pharmaceuticals, Langhorne, Pa), a gum containing aspirin, or a bite wafer (BW) reduced the pain and discomfort after archwire placement, no comparison of the effectiveness of either of these nonpharmacologic approaches and OTC medications has been published. This study was designed to compare the pain response during the first week after initial archwire placement in patients randomly assigned to 1 of 2 pain management groups: chewing on a BW as desired or taking an OTC analgesic according to the manufacturer’s directions.
Material and methods
Consecutive adolescent patients, 8 to18 years of age, accepted for treatment in the graduate orthodontic clinic at the University of North Carolina between August 2006 and November 2007 who met the inclusion and exclusion criteria ( Table I ) were invited to participate. Both 18-in and 22-in slot appliances were used, and a .014-in nickel-titanium was used as the first archwire with all teeth engaged. The study was approved by the Biomedical Institutional Review Board. Patients who completed the data collection protocol received $20 for their participation.
| Inclusion criteria |
| 1. Healthy adolescents (ages, 8-18) being treated at the University of North Carolina School of Dentistry Graduate Orthodontic Clinic |
| 2. Minimum weight of 88 lbs (FDA-approved OTC pediatric dosage labeling guidelines) |
| 3. Full band and bond in at least 1 arch |
| 4. Informed consent obtained from parent or guardian |
| Exclusion criteria |
| 1. Patient taking pain medications for chronic pain |
| 2. Medical condition that precluded the use of OTC pain medications |
| 3. Asthma |
| 4. Medications taken 3-4 days before the start of treatment |
| 5. Allergies to common OTC medications |
| 6. Oral surgery in the previous 4 weeks |
| No subject was excluded on the basis of sex or ethnicity. |
The study was designed as a stratified block randomized 2-group parallel clinical trial. Subjects were randomly assigned to either the OTC analgesic medication group or the BW group. Assignments were made with a computer-generated random assignment strategy that balanced groups with respect to sex (block size of 4). The assignments were placed in sealed envelopes and opened sequentially within strata.
Masking of the subjects was not possible because of the nature of the treatments. The specific pain management instructions given to each group were reviewed with the patient and parent before and immediately after initial archwire placement. Patients in the OTC group were instructed not to take medication before the prodedure, since the BW was not distributed before the appointment. The pain management instructions given to each group (patient and parent) were as follows. BW group: chew or bite on the wafer for 10 to 12 minutes within an hour after adjustment. Chew on the wafer as much as you want whenever you feel discomfort. Participants were given 2 of their choice of bubble gum, cherry, or grape flavored Dynaflex Therapy Wafers (DynaFlex, St. Ann, Mo). OTC group: take any OTC medicine of your choosing as needed for pain, according to the manufacturer’s directions.
Data by Polat et al were used to calculate the estimated sample size required for a noninferiority comparison of the 2 treatment groups. A noninferiority comparison was chosen because, from a clinical standpoint, there was no expectation that using a BW would provide better pain control than an analgesic. Twenty-five subjects per group, by using an unpaired t test approach with a 1-sided level of significance of 0.05, were estimated to provide approximately 80% power to detect an effect size difference of 0.70.
The subjects were asked to complete a questionnaire to rate their pain and the quality of their pain at 8 times after archwire placement: at 2 and 6 hours, at bedtime on the same day, 24 hours after the appointment, and at bedtime 2, 3, 5, and 7 days later. The subjects were given instructions on completion of the questionnaires before leaving the clinic and received a binder containing the questionnaire and a self-addressed, stamped envelope for return at the end of the protocol period.
Current pain and pain during 4 functions (biting on front and back teeth, chewing, and tapping teeth together 3 times) were assessed at each time point by using numerical rating scores from “no pain” (0) to “as bad as you can imagine” (10). Respondents also completed the Gracely box afferent (pain intensity) and sensory (unpleasantness of pain) scales at each time. Because the Gracely scales have not been used extensively with adolescents, each subject was asked to rank each group of words from the least (1) to the most intense or unpleasant (13) before archwire placement. The subject’s ranking of the words was used as the after archwire-placement numerical response to quantify the quality of the pain rather than the published adult rankings.
At bedtime each day, the subjects were asked to rate the average pain during the day using the same scale as current pain as well as the proportion of the day that pain was experienced (0 = no time to 10 = all day). The OTC treatment group was asked to document what pain medication had been used, the number of tablets, and the dose of each tablet. To assess the effectiveness of treatment, patients were asked how happy they were with their discomfort relief at the end of the week (0 = not happy to 10 = very happy). The BW treatment group was asked to document how many times the wafer had been used, the total number of minutes used, and its effectiveness for discomfort relief at the end of the week. If a subject in the BW group required rescue medication, he or she was asked to document what pain medication was used, the number of tablets, and the dose of each tablet. Subjects were encouraged not to take rescue medication and were asked to call the principal investigator (S.M.) if medication was taken.
Statistical analysis
The data for this project created a clustered data set with up to 8 responses per subject with unequal intervals between responses. To accommodate the likely correlation between responses over time and allow for the response variances to differ over time, a general linear mixed model (Proc Mixed, SAS, Cary, NC) with heterogeneous compound symmetric covariance matrix was fitted separately for each outcome variable (current pain, pain during function, affective pain, sensory pain) to compare the BW and OTC groups. Since all the covariance structures were not necessarily nested, the covariance structure was selected based on Akaike’s information criterion and the Bayesian information criterion, the most commonly used criteria for choosing among competing unnested covariance structures. The model controlled for the effect of sex, a design variable, and time. The interaction between group and time was also included to assess whether a differential pattern occurred over time in the 2 groups. The estimates and standard errors from the mixed models were used to test the hypothesis that the BW group, on average, was not inferior with respect to pain management to the OTC group. An intent-to-treat analysis was performed: subjects in the BW group who took rescue medication were included in the BW group for the primary analysis. The level of significance was set at P <0.05 for all analyses. An explanation of the calculation of the test statistic for the comparison of the 2 groups and the P values associated with the noninferiority hypothesis and the analysis of variance (ANOVA) table for all outcomes are given in the Appendix .
Results
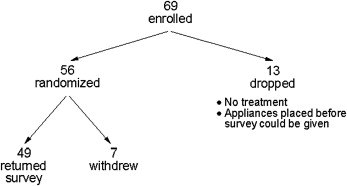
Sixty-nine adolescent subjects were enrolled. Thirteen were dropped ( Fig 1 ). Of the 56 subjects who were randomized, 87.5% successfully completed the survey ( Fig 1 ). The average age of those who did not complete the protocol was 13, and 4 of the 7 were girls. Two subjects had been assigned to the BW group and 5 to the OTC group. Sixty-nine percent of the participants who returned the survey were white, 16% were black, 4% were Hispanic, and 12% were other. The average age at enrollment for the 24 subjects randomized to the BW group was not significantly different from that of the 25 subjects in the OTC group (BW, mean = 13.6; SD = 2.0; OTC, mean = 13.7; SD = 1.7; unpaired t test; P = 0.90). The percentages of girls in the 2 groups were similar (BW, 54%; OTC, 52%; chi-square test, P = 0.88)
Patients in the OTC group used various medications: 40% an NSAID, 50% a non-NSAID, and 10% Percocet (Endo-Pharmaceuticals, Chadds Ford, Pa), oxycodone, and acetaminophen. These patients took an average of 1.5 tablets a day and recorded more use during the first 2 days: mean = 2.5 (SD = 1.5) and mean = 2.7 (SD = 2.3) tablets, respectively.
The BW was used an average of 3 times a day, with more use the first 2 days (mean = 4.7, SD = 6.4 and mean = 3.9, SD = 5.5), respectively, after archwire placement. Most patients used the BW for less than 20 minutes a day. Nine patients (37.5%) in the BW group used rescue medication at least once during the week. Five patients used rescue medication once, 2 patients used it twice, 1 used it 3 times, and 1 used it 4 times. All patients who used rescue medication took the first dose within the first 24 hours after archwire placement.
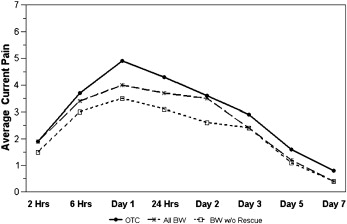
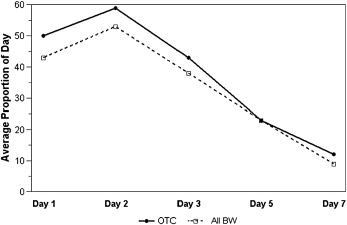
In both groups, the pain level peaked within the first 24 hours after archwire placement ( Table II ). The response pattern over time for current pain level ( Fig 2 ; P = 0.88), average pain during the day ( P = 0.91), and the proportion of the day with pain ( Fig 3 ; P = 0.85) was similar for the 2 groups. The average pain control in the BW group was not inferior to that in the OTC group for overall current pain level ( Fig 2 ; P = 0.82), average pain level during the day (P = 0.95), or the proportion of the day with pain ( Fig 3 ; P = 0.81). As expected, all 3 indicators of pain—current pain, average pain during the day, and proportion of the day with pain—decreased significantly over time ( P <0.0001).
| Current pain | Average daily pain | Proportion of day with pain | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OTC | BW | OTC | BW | OTC | BW | |||||||
| Time | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 2 h | 1.9 | 2.1 | 1.9 | 2.3 | ||||||||
| 6 h | 3.7 | 2.9 | 3.4 | 2.6 | ||||||||
| Day 1 | 4.9 | 2.6 | 4.0 | 2.7 | 5.2 | 2.2 | 4.2 | 2.5 | 5.0 | 2.6 | 4.3 | 2.4 |
| 24 h | 4.3 | 2.3 | 3.7 | 2.3 | ||||||||
| Day 2 | 3.6 | 2.3 | 3.5 | 2.6 | 5.3 | 2.6 | 4.3 | 2.5 | 5.9 | 3.2 | 5.3 | 3.3 |
| Day 3 | 2.9 | 2.4 | 2.4 | 2.1 | 4.2 | 3.0 | 3.5 | 2.5 | 4.3 | 2.9 | 3.8 | 2.7 |
| Day 5 | 1.6 | 1.8 | 1.2 | 1.2 | 2.2 | 2.1 | 1.4 | 1.5 | 2.3 | 2.6 | 2.3 | 2.6 |
| Day 7 | 0.8 | 1.5 | 0.4 | 0.9 | 1.3 | 2.0 | 0.8 | 1.2 | 1.2 | 1.7 | 0.9 | 1.4 |
The pattern of pain reported over time was similar for the 4 functions (biting on front and back teeth, chewing, and tapping teeth together) ( Table III ; P = 0.33), although the overall pain levels differed significantly among the 4 functions ( P <0.0001). Pain experienced from biting on the back teeth was significantly lower than that from biting on the front teeth ( Fig 4 , A ; P <0.0001), and chewing was significantly more painful than tapping the teeth together ( Fig 4 , B ; P <0.003).
| Biting on front teeth | Biting on back teeth | Chewing | Teeth tapping | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OTC | BW | OTC | BW | OTC | BW | OTC | BW | |||||||||
| Time | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 2 h | 2.7 | 2.9 | 2.5 | 3.1 | 2.5 | 2.9 | 2.3 | 2.6 | 3.7 | 3.5 | 2.3 | 2.5 | 2.8 | 3.2 | 1.6 | 2.1 |
| 6 h | 4.8 | 2.9 | 3.2 | 2.8 | 3.4 | 3.3 | 3.3 | 2.8 | 4.3 | 3.5 | 4.2 | 3.0 | 4.6 | 3.6 | 3.6 | 3.0 |
| Day 1 | 6.1 | 2.9 | 4.4 | 2.5 | 4.4 | 3.2 | 3.8 | 3.1 | 6.2 | 3.4 | 4.7 | 3.0 | 5.8 | 3.3 | 3.5 | 2.6 |
| 24 h | 5.7 | 2.6 | 4.8 | 2.4 | 4.0 | 3.2 | 3.4 | 2.7 | 5.5 | 2.8 | 5.2 | 3.3 | 6.0 | 2.9 | 3.8 | 2.9 |
| Day 2 | 5.1 | 3.0 | 4.1 | 3.0 | 3.9 | 3.3 | 3.0 | 2.6 | 5.6 | 3.1 | 4.6 | 3.1 | 5.4 | 3.3 | 3.9 | 3.0 |
| Day 3 | 4.4 | 3.0 | 3.2 | 2.2 | 3.3 | 3.3 | 2.3 | 2.2 | 4.7 | 3.0 | 3.5 | 3.0 | 4.6 | 3.2 | 2.8 | 2.9 |
| Day 5 | 2.6 | 2.5 | 2.1 | 2.2 | 1.5 | 2.0 | 1.1 | 1.2 | 2.2 | 2.4 | 1.9 | 1.8 | 2.2 | 2.4 | 1.5 | 2.2 |
| Day 7 | 2.0 | 2.5 | 0.9 | 1.4 | 1.2 | 2.0 | 0.4 | 0.9 | 1.6 | 2.2 | 0.6 | 1.2 | 1.3 | 1.9 | 0.6 | 1.4 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


