Chapter 4
Subgingival Microbes
Abstract
This chapter describes the cell and colony morphology, cultural and biochemical characteristics of oral microbes that are mainly isolated from subgingival plaques, including gram-positive bacteria of the genera Enterococcus, Eubacterium, Peptostreptococcus, and Propionibacterium, as well as gram-negative bacteria of the genera Bacteroides, Capnocytophaga, Eikenella, Fusobacterium, Helicobacter, Aggregatibacter, Prevotella, Porphyromonas, and Treponema.
Keywords
Aggregatibacter; Bacteroides; Capnocytophaga; Eikenella; Enterococcus; Eubacterium; Fusobacterium; Helicobacter; Peptostreptococcus; Prevotella; Porphyromonas; Propionibacterium; Subgingival; Treponema
4.1. Gram-Positive Bacteria
4.1.1. Enterococcus
Enterococci are facultative gram-positive cocci and belong to Lancefield group D. Enterococci faecalis is also called Streptococcus faecalis and is the most common species in the genus Enterococcus. In recent years, it has been closely studied due to its high detection rate in infected root canals.
4.1.1.1. Enterococcus faecalis
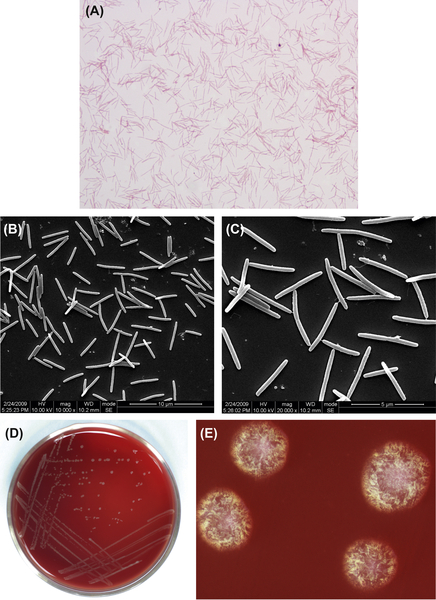
E. faecalis cells are gram-positive, oval (0.5–1 μm in diameter), and nonmotile. Most cells are arranged in pairs or as short chains (Figure 4.1(A)). Cellular morphology by SEM is shown in Figure 4.1(B). The GC content of its DNA is 33.5%. The type strain is NCTC775 (ATCC19433, NCDO5681).
E. faecalis is a facultative anaerobe. Cells of this species can form smooth, nontransparent, white or creamy, spherical colonies on common nutrient agar plates (Figure 4.1(C)). However, colonies formed on PSE agar plates containing cholate, esculin, and sodium azide (the selective agar medium for Pfizer Enterococci) are brownish black with brown aureole (Figure 4.1(D)). This can be used as a characteristic to distinguish E. faecalis from other bacteria. Colonies observed by stereomicroscope are shown in Figure 4.1(E).
E. faecalis can ferment most carbohydrates. The main acid produced by glucose fermentation is lactic acid. E. faecalis can also hydrolyze arginine to produce ammonia.
4.1.2. Eubacterium
Eubacterium is a genus of gram-positive nonsporulating strictly anaerobic bacilli. The name of the genus is still disputed. Bergey’s Manual of Systematic Bacteriology vol. 2 (1986) points out that the Greek prefix eu means good, useful, rather than true. Therefore, the author believes that Eubacterium is the more appropriate name. Currently, bacteria species detected in the oral cavity that belong to this genus include Eubacterium alactolyticum, Eubacterium saburreum, Eubacterium lentum, Eubacterium limosum, Eubacterium nodatum, Eubacterium brachy, Eubacterium timidum, Eubacterium saphenus, and Eubacterium minutum.
Cells can be homogeneous or polymorphous rod-shaped. No spores are produced. Cells are gram-positive, but Gram staining old cultures and cultures that have produced acid in the culture medium will yield negative results.
Eubacteria are strictly anaerobic. Culturing cells can be difficult due to this bacteria’s strict anaerobic demands, and some strains can only grow in prereduced medium. The optimum growth temperature is 37 °C, while the optimum pH is 7.0.
Eubacteria are chemoheterotrophs and produce energy from mixed organic acids produced by carbohydrates or protein metabolism. These mainly consist of butyric acid, acetic acid, and formic acid.
Most Eubacteria from the oral cavity are relatively biochemically inactive. In most cases, cells test negative for catalase and do not hydrolyze hippurate. Carbohydrate fermentation, indole production, nitrate reduction, esculin hydrolysis, and other biochemical tests can help differentiate the different species in the genus.
Eubacteria mainly colonize the saliva and plaque as a member of the normal oral microflora. Eubacterium lentum and E. limosum can be detected in the oral cavity. Eubacterium nodatum, E. brachy, E. timidum, E. saphenus, and E. minutum are new species isolated from subgingival plaque of patients with periodontitis and are considered as potential periodontal pathogens. The GC content in Eubacterium DNA is 30–55% (analyzed by Tm), and the percentage in the type species is 47% (by Tm).
4.1.2.1. Eubacterium lentum
In 1999, Kageyama et al. called for a change in the classification of E. lentum and proposed that it be grouped with Eggerthella lenta, the type species of genus Eggerthella.
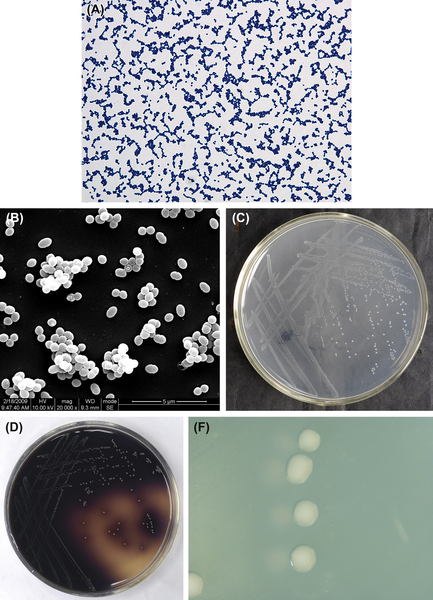
Figure 4.1 (A) Cells of E. faecalis (gram-positive cocci). (B) Cells of E. faecalis (SEM). (C) Colonies of E. faecalis (common nutrient agar plate). (D) Colonies of E. faecalis (PSE agar plate). (E) Colonies of E. faecalis (stereomicroscope).
E. lentum is a gram-positive, irregular, nonsporulating, strictly anaerobic bacillus (Figure 4.2(A) and (B)). As a strict anaerobe, most strains can grow between 30 °C and 45 °C, while some strains can grow at 25 °C. Arginine can promote bacterial growth. Characteristic colonies are shown in Figure 4.2(C) and (D).
E. lentum does not ferment carbohydrates. The cells do not hydrolyze esculin, hippurate, and gelatin. Ammonia can be produced from arginine, and H2O2 can be produced from agar medium containing 1% arginine. Under anaerobic conditions, the bacteria can produce H2S in the beveled bottom of triple sugar iron agar but cannot produce H2S in SIM culture medium. The GC is 62% in DNA, and the type strain is JCM 9979 (= DSM2243 = ATCC25559 = NCTC11813).
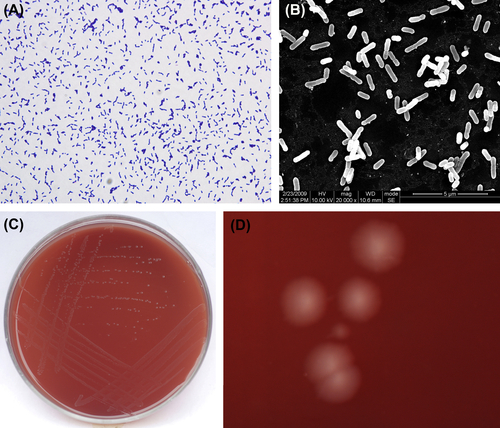
Figure 4.2 (A) Eubacterium lentum cells (Gram stain). (B) E. lentum cells (SEM). (C) E. lentum colonies (BHI blood agar). (D) E. lentum colonies (stereomicroscope). E. lentum on horse blood agar forms surface colonies that are 0.5–2 mm in diameter, rounded, convex or convex, low, dim, and dark or lustrous, translucent or opaque, smooth, wedge-shaped, or neat-edged. A striped appearance can be observed under incident light.
4.1.3. Peptostreptococcus
Peptostreptococcus is the most common gram-positive anaerobic coccus in the human oral cavity and in the clinic. Peptostreptococcus anaerobius and Peptostreptococcus micros are the most commonly encountered species in this genus.
4.1.3.1. Peptostreptococcus anaerobius
The cells of P. anaerobius are gram-positive (Figure 4.3(A)), spherical, approximately 0.5–0.6 μm in diameter, and arranged in pairs or chains. Cells in early cultures have been observed to form long chains. Cells observed by SEM are shown in Figure 4.3(B). The GC content of the P. anaerobius genome is 33–34%, and its type strain is ATCC27337.
The optimal temperature for P. anaerobius growth is 37 °C, and cells of this species do not grow well at 25 °C or 30 °C, and do not grow at all at 45 °C. Growth is stimulated by 0.02% polysorbate-80. P. anaerobius cells form pinpoint-like or rounded (about 1 mm in diameter), raised, white, glossy, nontransparent colonies with a smooth surface, without hemolytic zone (Figure 4.3(C)). Colonies formed on the surface of BHI supplemental medium without addition of blood are gray. Broth cultures of P. anaerobius are usually not muddy, and granulated or viscous precipitates can be observed. Colonies observed by stereomicroscope are shown in Figure 4.3(D).
P. anaerobius is relatively biochemically inactive, and cells usually do not ferment carbohydrates. The main acidic metabolic end products in PVG liquid culture of the type strain are acetic acid, isobutyric acid, butyric acid, isovaleric acid, and isocarproic acid. P. anaerobius can produce CO2 and H2 from pyruvates under anaerobic conditions. In deep glucose agar, P. anaerobius can produce a large amount of gas and generate ammonia from peptone.
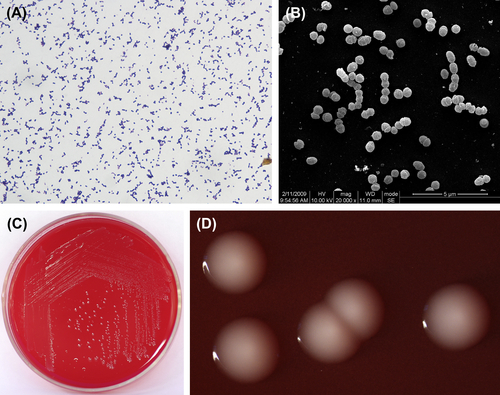
Figure 4.3 (A) Cells of P. anaerobius (Gram stain). (B) Cells of P. anaerobius (SEM). (C) Colonies of P. anaerobius (BHI blood agar plate). (D) Colonies of P. anaerobius (stereomicroscope).
Dental plaque and gingival sulcus are the main habitats for P. anaerobius in the oral cavity. Moreover, P. anaerobius is often detected in clinical samples of periodontitis, pulpitis, and pericoronitis.
4.1.4. Propionibacterium
Propionibacterium is a genus of gram-positive polymorphic bacilli that do not sporulate. The genus can be divided into two groups: one that lives on the skin, including inside the oral and intestinal tracts, named the sores and blisters group or P. acnes; and another that lives in dairy products, cheese or green fodder named dairy group or typical propionibacteria.
The cells are polymorphic in form, many have two rounded ends, and others are shaped like Corynebacterium diphtheria, with one rounded end and one tapered end. Cells are 0.5–0.8 μm in diameter, 1–5 μm in length, and form two branches or branched rods. Cocci are observed in old cultures, arranged as single cells, in pairs, in chains, or in “Y”- or “V”-shaped branched chains. Cells are gram-positive, but some cells can also stain gram-negative.
Members of this genus are anaerobic or microaerophilic bacteria. The highest rate of growth takes place 48 h after inoculation. The Propionibacteria are chemoheterotrophic bacteria that need a complex nutritional medium such as BHI agar in order to be cultured. Most species can grow in dextrose broth containing 20% bile or 6.5% NaCl. Propionibacterium acnes colonies on the surface of agar can produce colorful pigmentation including white, gray, pink, red, or yellow.
The main acid metabolites are propionic acid and acetic acid when cultured in PYG broth. The production of a significant quantity of propionic acid is the identifying feature of this genus when attempting to differentiate them from other gram-positive nonsporulating anaerobic bacteria. They can also produce some isovaleric acid, formic acid, succinic acid, and lactic acid.
All the species in this genus can use glucose to produce acid. They test positive for catalase. Species of Propionibacterium are distinguished using indole production, nitrate test, esculin hydrolysis, gelatin liquefication, and other biochemical tests such as the fermentation of sucrose, maltose, and mannitol.
The GC content of Propionibacterium DNA is 53–57% by Tm method. The type species is P. freudenreichii.
4.1.4.1. Propionibacterium acnes
P. acnes is a gram-positive irregular bacillus (Figure 4.4(A), (B), and (C)). It is either anaerobic or microaerophilic. A medium with low redox potential is required for primary cultures. Characteristic colonies are shown in Figure 4.4(D) and (E). Culture in dextrose broth is cloudy or clear with fine granular precipitates. In old broth, the culture exhibits light red precipitation. The final pH value of a culture is 4.5–5.0 in PYG broth. The terminal acid metabolites are propionic acid and acetic acid.
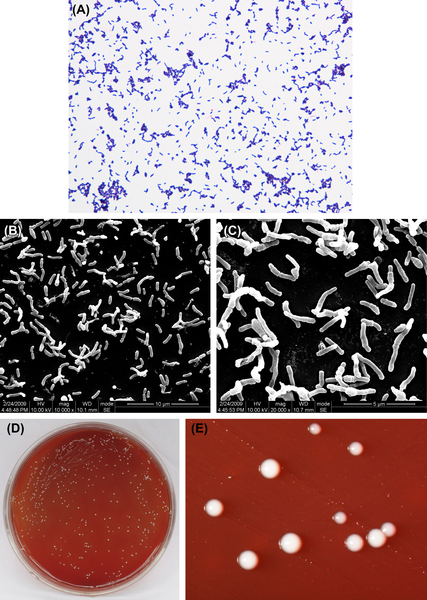
Figure 4.4 (A) Propionibacterium acnes cells (Gram stain). (B) P. acnes cells (SEM). (C) P. acnes cells (SEM). P. acnes cells are polymorphic but are mainly thin and long rod-shaped cells. Sero variant type II cells are mainly spherical with no spores. Cells stain gram-positive, but some can stain gram-negative. (D) P. acnes colonies (BHI blood agar). (E) P. acnes colonies (stereomicroscope). P. acnes can generate pinprick-sized colonies to colonies 0.5 mm in diameter, white or gray, glossy, translucent or opaque, pad-shaped or neat-edged colonies 2–3 days postinoculation on the surface of horse blood or rabbit blood agar. On rabbit blood agar, 68% of Sero variant type I strains can generate hemolytic reaction while Sero variant type II strains cannot.
P. acnes is part of the normal microflora, but the number of cells varies greatly between different individuals. P. acnes can be separated from the secretions from acne, wounds, blood, pus, and intestinal contents. The GC content of the bacterial DNA is about 57–60% (by Tm method). The type strain is ATCC6919 (NCTC737).
4.2. Gram-Negative Bacteria
4.2.1. Bacteroides
Bacteroides is a genus of gram-negative, obligate anaerobic, nonsporulating bacilli that belong to the family Bacteroidaceae. Members of this genus are chemoheterotrophs and can use carbohydrates, peptone, and other intermediate bacterial metabolites.
Bacteroides are naturally found in the mouth, tongue, intestinal tract, and vagina. All strains that can tolerate bile (20% oxgall salts) fall collectively into the B. fragilis group, including B. fragilis, B. thetaiotaomicron, etc. that are detected in cultures from appendicitis, peritonitis, and cervicitis clinical specimens. Other species that cannot tolerate bile and that produce melanin were reclassified to the genera Porphyromonas and Prevotella.
Cells measure 0.8–1.8 μm × 0.8–1.6 μm when grown in glucose broth, often showing visible vacuoles or darker stain at the ends. Cells are arranged singly or in pairs with no spores. Many strains are encapsulated.
4.2.1.1. Bacteroides fragilis
B. fragilis are gram-negative bacilli (Figure 4.5(A), (B), and (C)). They are obligate anaerobes and grow well in nutrient agar supplemented with chlorinated hemoglobin and vitamin K1. Growth is inhibited by 20% bile. Optimum growth takes place at pH 7.0. Characteristic colonies are shown in Figure 4.5(D) and (E).
The cells mainly produce succinic acid and acetic acid in PYG liquid medium, but lactic acid, propionic acid, isovaleric acid, isobutyric acid, formic acid, benzene, and acetic acid are also produced. This species is biochemically active and is capable of fermenting glucose, lactose, maltose, fructose, raffinose, and other carbohydrates, hydrolyzing esculin. B. fragilis tests negative with the indole test.
The percent GC in B. fragilis DNA is 41–44% (using the Tm method). The type strain is ATCC25285 (NCTC9343).
4.2.1.2. Bacteroides thetaiotaomicron
This species is gram-negative (Figure 4.6(A) and (B)) and its morphology under SEM is shown in Figure 4.6(C) and (D).
B. thetaiotaomicron are obligate anaerobes and have similar growth requirements and characteristics as B. fragilis. Characteristic colonies are shown in Figure 4.6(E) and (F).
Cells exhibit active biochemistry and are able to ferment glucose, lactose, maltose, fructose, raffinose, arabinose, cellobiose, rhamnose, and other carbohydrates. Cells can hydrolyze esculin and test positive for indole. Cells grow well in medium containing 20% bile. The genomic GC content is 40–43% (by Tm). The type strain is ATCC29148 (NCTC10582).
4.2.2. Capnocytophaga
Capnocytophaga are gram-negative, facultative anaerobic bacteria. They were the earliest bacteria to be isolated and named from the human subgingival plaque. Common Capnocytophaga species are: Capnocytophaga ochracer, Capnocytophaga sputigena, Capnocytophaga gingivalis, Capnocytophaga granulose, and Capnocytophaga heamolytica.
Capnocytophaga cells are 0.42–0.6 μm × 2.5–2.7 μm in size and shaped like bent rods or filaments, usually with rounded or slightly pointed ends. The length of the cells varies. In liquid culture, cells are polymorphic or take on a long, filamentous morphology, and tight clumps can be observed. The bacteria produce no capsule and no sheath. They do not form spores, have no flagella, but have sliding motility.
Capnocytophaga are facultative anaerobes but do not grow under aerobic conditions. Cultures grow well in a CO2-added anaerobic environment. Primary cultures should be performed in an aerobic environment with CO2 added.
Species in this genus often form colonies of wet, thin, flat, diffuse growth with ragged edges on TS blood agar and BHI blood agar. After 24 h incubation at 35–37 °C, the size of colonies are like pinpricks. After incubation for 48–96 h, colonies become 2–4 mm in diameter and take on the appearance of bumps. Some colonies may become recessed into the agar. Aside from hemolytic Capnocytophaga (which produces β-hemolysis), other species are not hemolytic on blood agar. The concentration of agar in the medium affects the force of sliding motility. Capnocytophaga cultures can produce a special smell, similar to caramel or a bitter almond flavor.
Colonies on the agar surface can produce white to pink or orange-yellow pigmentation. Centrifuged cells appear to be an orange-yellow clump.
Capnocytophaga do not produce indole, can ferment glucose, lactose, maltose, mannose, and sucrose acid, and do not ferment mannitol and xylose. They can hydrolyze esculin and test negative for catalase and oxidase, while testing positive for ONPG and benzidine. Nitrate reduction, dextran hydrolysis, starch or gelatin hydrolysis and other biochemical tests can help identify this genus of bacteria.
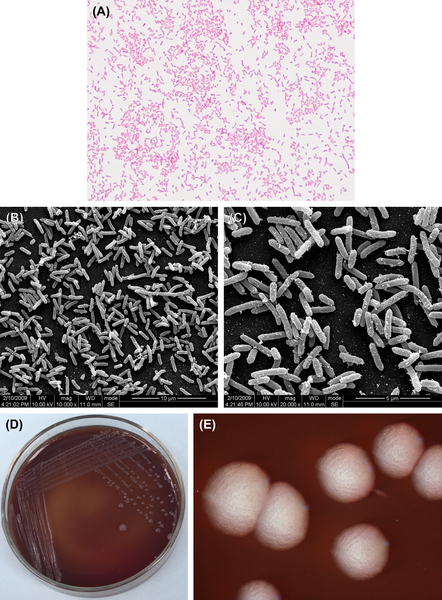
Figure 4.5 (A) Bacteroides fragilis (Gram stain). (B) B. fragilis (SEM). (C) B. fragilis (SEM). B. fragilis appears rounded on both ends, with visible vacuoles or darker stain at the ends. The cells are approximately 0.8–1.8 μm × 1.6–0.8 μm when grown in glucose broth. The cells can be single or in pairs, and they form no spores and are gram-negative. (D) B. fragilis colonies (BHI blood agar). (E) B. fragilis colonies (stereomicroscope). B. fragili can form gray colonies about 1–3 mm in diameter. The colonies are rounded, smooth, translucent or semitransparent on blood agar.
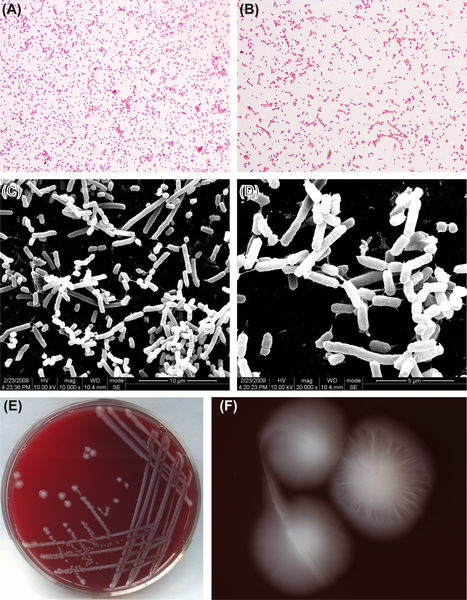
Figure 4.6 (A) Bacteroides thetaiotaomicron cells (Gram stain). (B) B. thetaiotaomicron cells (Gram stain). (C) B. thetaiotaomicron cells (SEM). (D) B. thetaiotaomicron cells (SEM). B. thetaiotaomicron appears spherical or rounded on both ends, with visible dark stain at the ends. Cells are 0.7–1.1 μm × 1.3–8.0 μm when grown in glucose broth, and are arranged as single cells or in pairs. Cells stain gram-negative. (E) B. thetaiotaomicron colonies (BHI blood agar). (F) B. thetaiotaomicron colonies (stereomicroscope). B. thetaiotaomicron colonies are about 1–3 mm in diameter. They form bumpy, glossy, soft, white, round colonies on blood agar. An orange peel-like appearance can be observed on the colony surface under stereomicroscope.
A member of the normal microflora of humans and primates, this genus is mainly found to colonize the oral cavity. They are common oral bacteria and can be obtained from various parts of the oral cavity, including plaque, gingival sulcus, saliva and sputum, and throat specimens. These bacteria are often detected in mixed bacterial infections, such as juvenile periodontitis, infected root canal, dry socket after tooth extraction, oral ulcers, and other clinical specimens, and can also be isolated from bacteremia, soft tissue infections, injuries and abscesses at various locations, cerebrospinal fluid, vaginal, cervical, and amniotic fluid, trachea, and eyes.
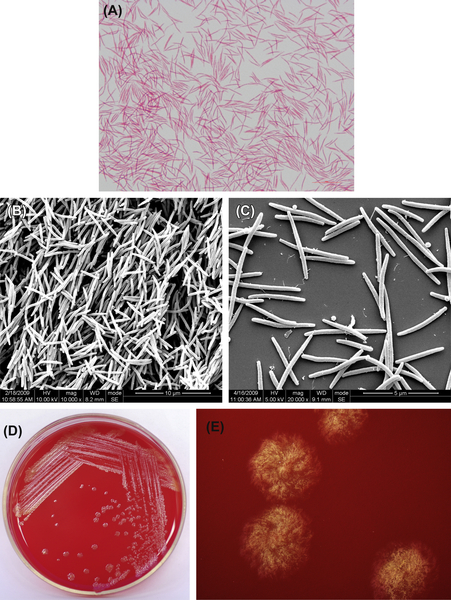
Figure 4.7 (A) Cells of C. gingivalis (Gram stain). (B) Cells of C. gingivalis (SEM). (C) Cells of C. gingivalis (SEM). Cells of C. gingivalis are fusobacterium-shaped, and the ends are usually rounded. Cells are often arranged in an orderly manner and stain negative by Gram stain. (D) Colonies of C. gingivalis (BHI blood agar). (E) Colonies of C. gingivalis (stereomicroscope). Colonies of C. gingivalis on BHI blood agar are irregular, gray colonies, with ragged edges. Typical hair-like diffuse colonies can be seen under the stereomicroscope.
The GC content of Capnocytophaga genomic DNA is 33–41% (by Tm method). The type species is yellowish Capnocytophaga.
4.2.2.1. Capnocytophaga gingivalis
This gram-negative bacterium is shown in Figure 4.7(A), (B), and (C). Characteristic colonies are shown in Figure 4.7(D) and (E).
C. gingvalis does not ferment lactose, galactose, amygdalin, salicin, cellobiose, esculin, and glycogen. It also does not hydrolyze starch, dextran, and gelatin. Only 8% of the strains can reduce nitrate.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses